Uses and compositions for treatment of ankylosing spondylitis
a technology of ankylosing spondylitis and compositions, applied in the direction of drug compositions, antibody medical ingredients, peptides, etc., can solve the problems of fatigue and pain associated with use, impairment of health-related quality of life, and eventual loss of spinal mobility, so as to improve the fatigue and pain associated, safe and effective treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Adalimumab in the Treatment of Active Ankylosing Spondylitis: Results of an Open-Label, 52-Week Trial
[0203]Tumor necrosis factor (TNF) antagonists infliximab and etanercept have shown efficacy in the treatment of ankylosing spondylitis (AS). Adalimumab (Abbott Laboratories) is a fully human, anti-TNF monoclonal antibody that reduces the signs and symptoms and progression of disease of rheumatoid arthritis and has been evaluated in AS over 20 weeks.
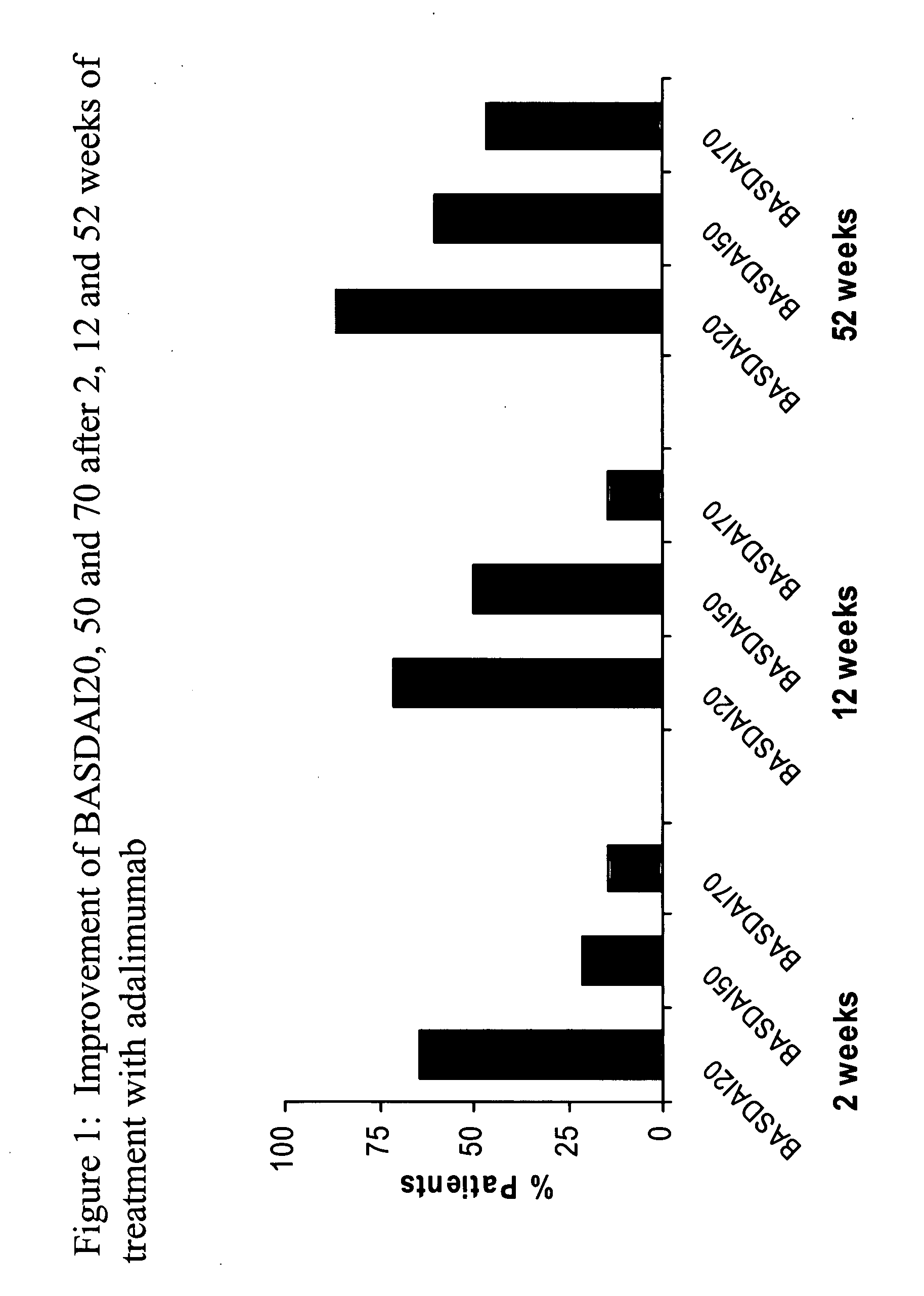
[0204]The objective of this study was to examine the potential therapeutic effects of adalimumab in NSAID-refractory AS patients who were treated for 52 weeks (Ann Rheum Dis 2005; 64(Suppl III):316). To further this objective, fifteen patients were enrolled (patient characteristics are detailed in Table 1). All patients suffered from spinal pain, and 4 patients also had peripheral arthritis. Adalimumab 40 mg was administered subcutaneously every other week (eow). Clinical outcome assessments included disease activity (BASDAI), function (BA...
example 2
Adalimumab Reduces Fatigue in Patients with Active Ankylosing Spondylitis (AS)
[0209]Fatigue, defined as enduring, subjective sensation of generalized tiredness or exhaustion, has been increasingly recognized as an important outcome measure in AS (Dagfinrud et al. Arth Rheum 2005; 53(1):5-11; Jones S D et al. J Rheumatol 1996; 23(3):487-90; Haywood H L et al. Rheumatol 2002; 41:1295-1302; Ward M M. Arth Care Res 1999; 12:247-55; Van Tubergen et al. Arth Rheum 2002; 27(1):8-16). It has been reported that 65% of people living with AS describe fatigue as a major symptom from time to time (Jones S D et al. J Rheumatol 1996; 23(3):487-90). The objective of this study was to evaluate the impact of adalimumab therapy (a TNF antagonist) on fatigue in active AS patients.
Overview
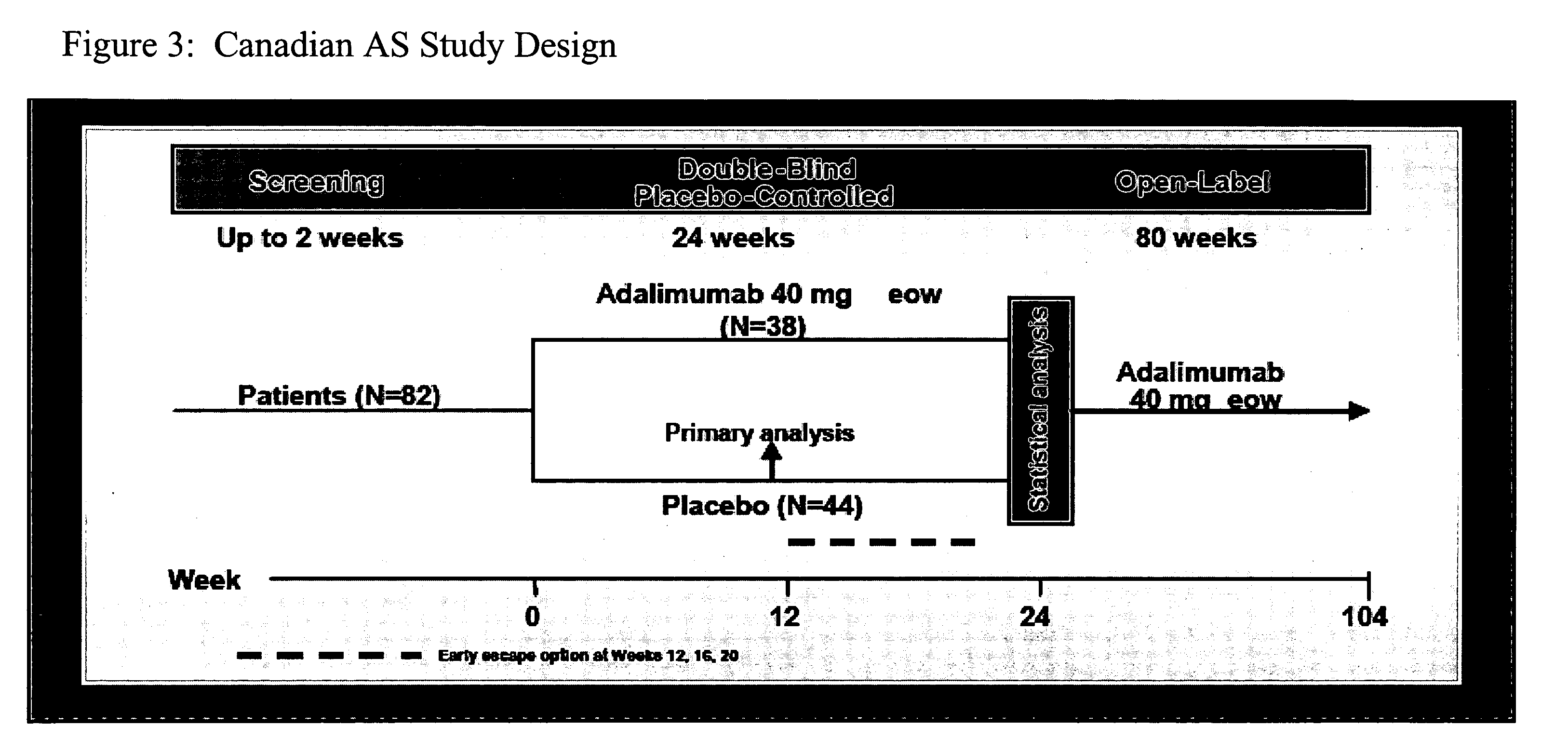
[0210]This phase III, double-blind, randomized, placebo-controlled trial was conducted at 11 sites in Canada (study design is shown in FIG. 3). The study enrolled active AS patients with an inadequate response to at le...
example 3
Major Clinical Response and Partial Remission in Ankylosing Spondylitis Subjects Treated with Adalimumab: Study H
[0221]Ankylosing Spondylitis (AS) is a common inflammatory rheumatic disease that produces progressive spinal stiffness and restriction of mobility. Tumor necrosis factor (TNF) is thought to play a major role in the pathogenesis of AS. No trial of a disease-modifying antirheumatic drug (DMARD) has yielded consistent positive results for the treatment of AS.
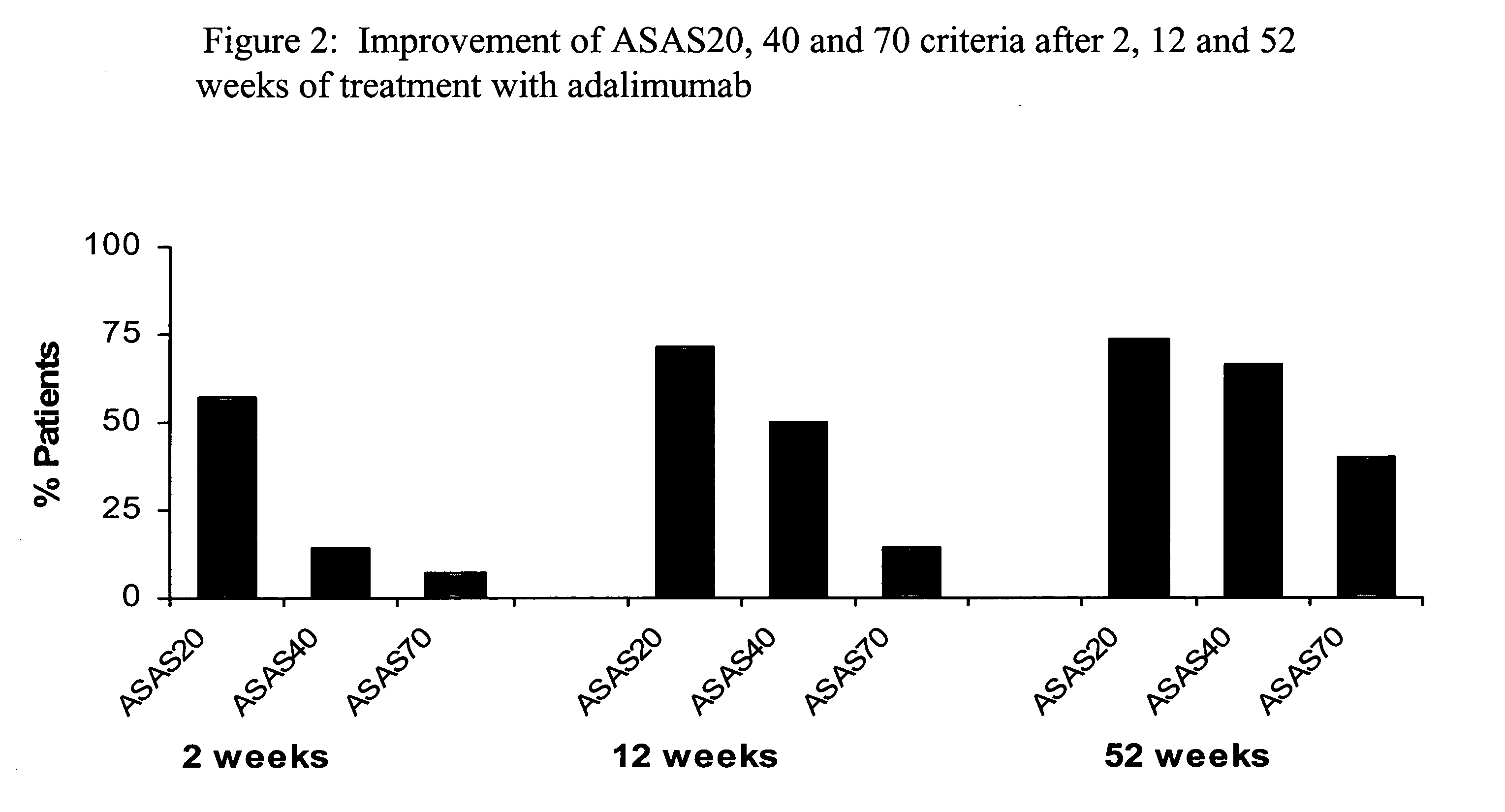
[0222]Adalimumab is a fully human monoclonal antibody targeting TNF, currently approved for the treatment of rheumatoid arthritis and psoriatic arthritis in the US and Europe, and currently pending approval from the FDA and EMEA for AS. The objective of the study described herein was to investigate the ability of adalimumab to effect a major clinical response and partial remission in subjects with ankylosing spondylitis. Partial remission is defined as a value of <20 on a 0-100 VAS scale in each of the 4 ASAS domains: P...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time period | aaaaa | aaaaa |
| time period | aaaaa | aaaaa |
| time period | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com