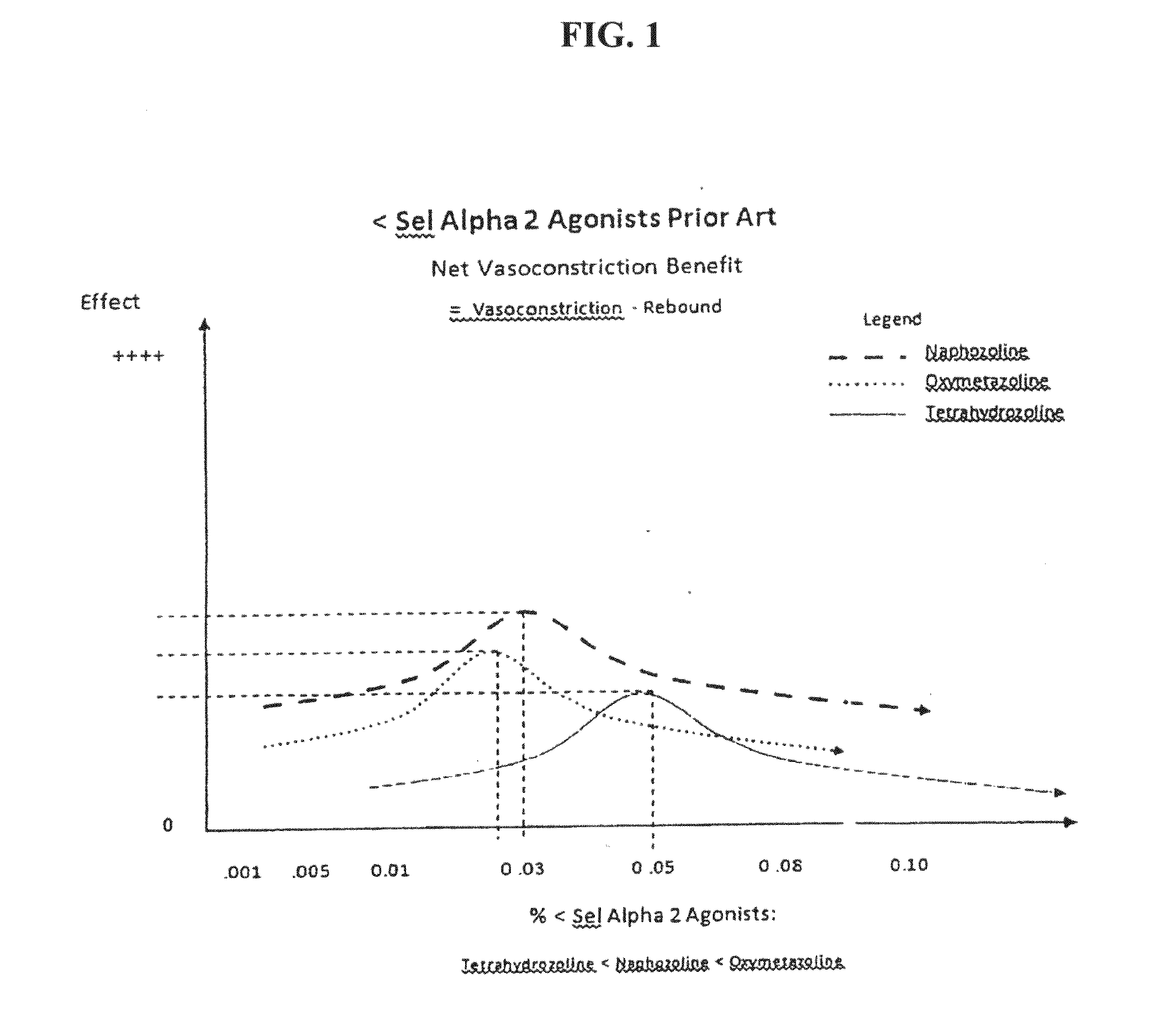
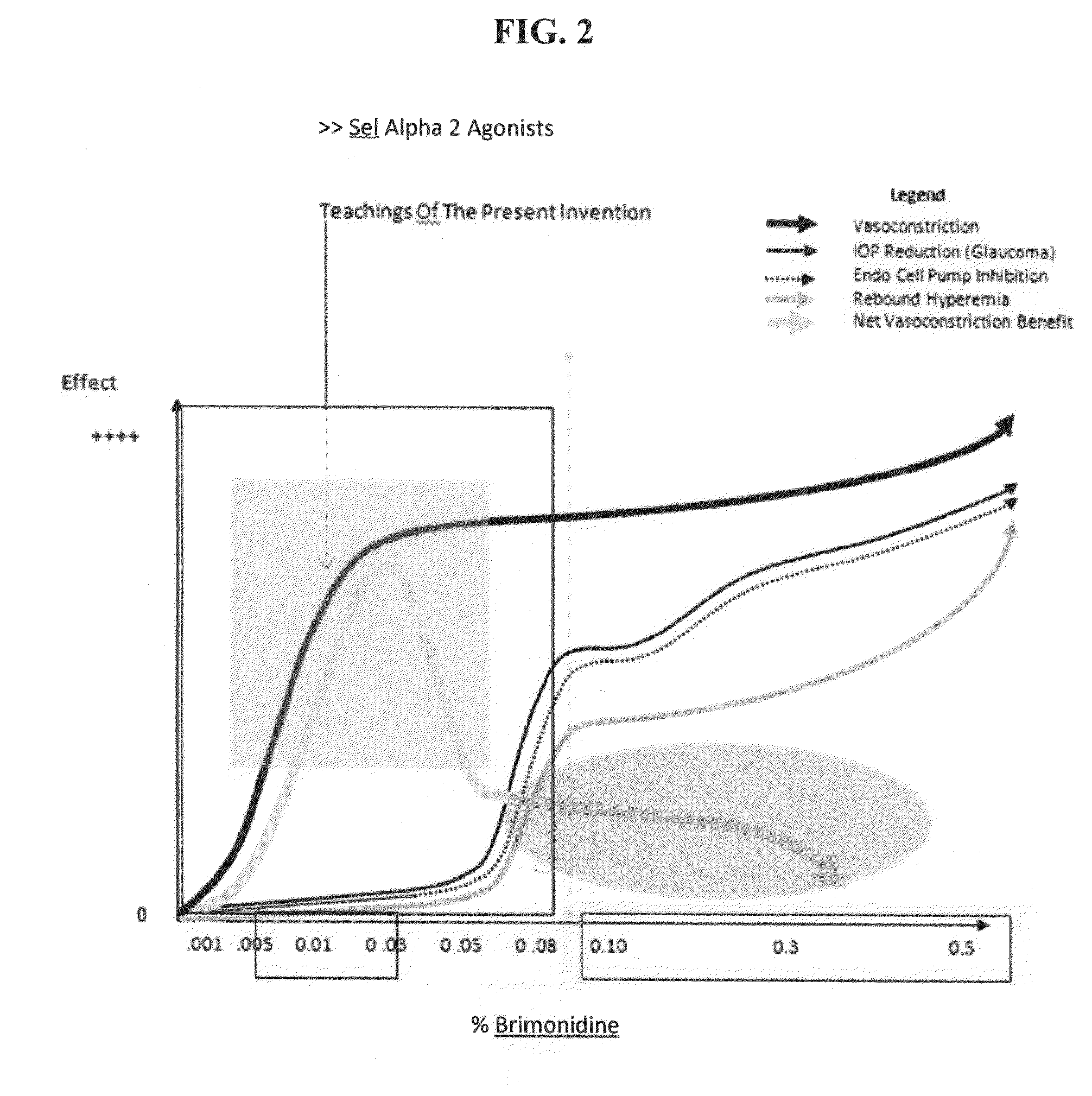
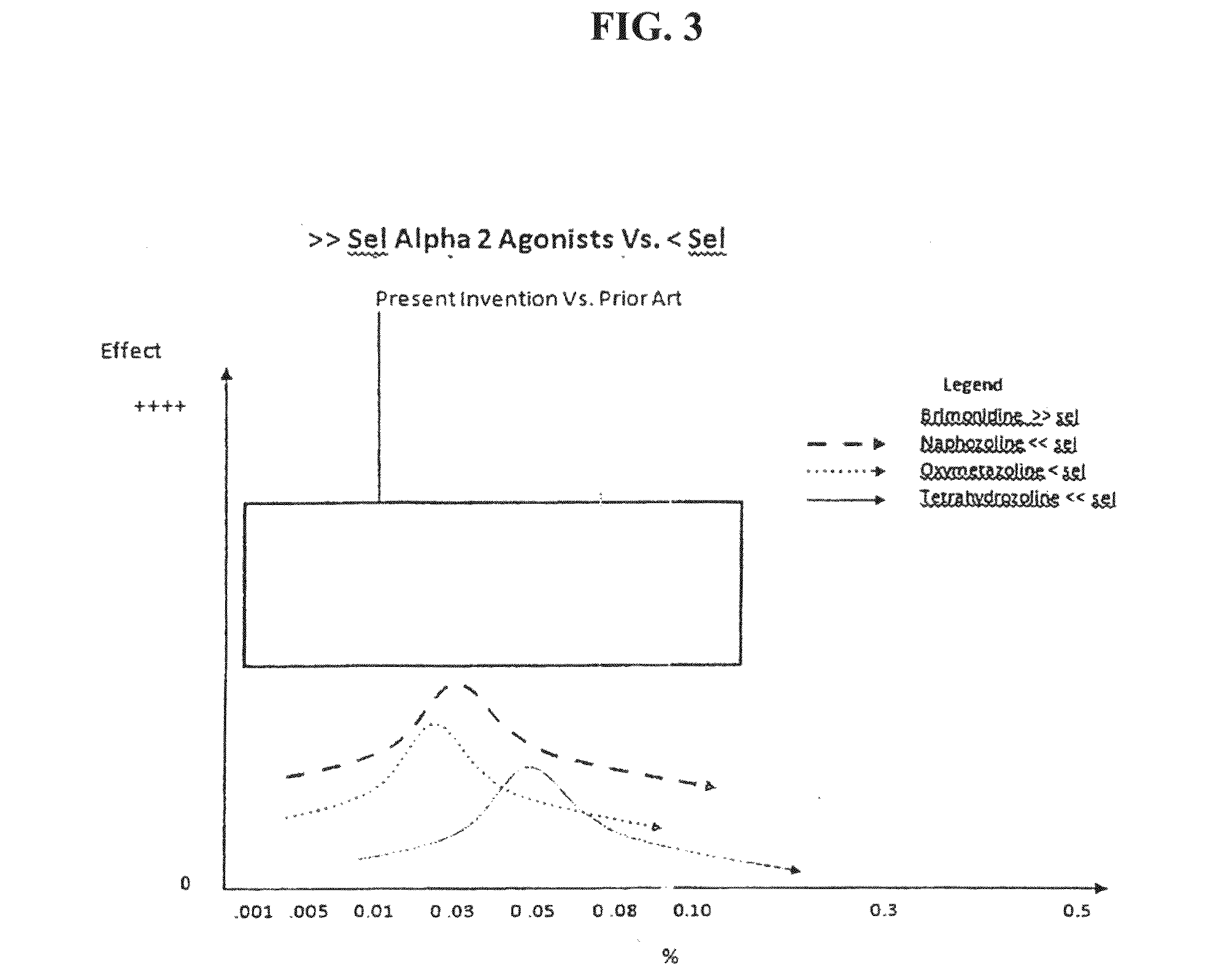
Composition and methods for treating allergic response
a technology for allergic reactions and compositions, applied in the direction of biocides, drug compositions, aerosol delivery, etc., can solve the problems of rebound hyperemia, drug side effects, and high incidence of rebound hyperemia, and achieve the effect of reducing hyperemia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0178]In this Example, a patient was treated with brimonidine at claimed concentrations and prior art compositions of tetrahydrozoline, oxymetazoline and naphazoline.
[0179]The results clearly demonstrate significant scleral whitening brightening effects of treatment with brimonidine as compared with treatment with prior art compositions.
[0180]The results are shown in FIGS. 4A through 4E.
[0181]FIG. 4A shows the base line for both eyes.
[0182]FIG. 4B shows a comparison after 180 minutes, where the right eye has been treated with tetrahydrozoline at 0.05% and the left eye was treated with brimonidine at 0.01%
[0183]FIG. 4C shows a comparison four hours after baseline (FIG. 4A), where the right eye has been treated with oxymetazoline at 0.025% and the left eye was treated with brimonidine at 0.02%
[0184]FIG. 4D shows a comparison where after a further four hours, the right eye has been treated with naphazoline at 0.033%; and the left eye was treated with brimonidine at 0.02%.
[0185]FIG. 4E ...
example 2
Lasik Prophylaxis
[0188]Baseline:
[0189]Treatment of 200 patients via the Intralase femtosecond laser with no pretreatment for vasoconstriction—significant postoperative hyperemia and conjunctival hemorrhage with @ 15% petichial or larger hemorrhage when patients were seen postoperative day 1, 25% 1+(14) hyperemia first hour+; 50% 2.5+hyperemia first how+; 25% 3+hyperemia first hour+. Flap dislocation rate: <0.1%.
[0190]Treatment Group 1:
[0191]Naphcon-A® (Alcon, Inc; active ingredients: naphazoline hydrochloride 0.25% and pheniramine maleate 0.3%; preserved with benzalkoniurnm chloride) was used on a second group of 50 patients (85 procedures), 12% petichial or larger hemorrhage. 35% 1+hyperemia; 35% 2+hyperemia; 15% 2.5+hyperemia; 15% 3+hyperemia. Some clinical benefit noted. Flap dislocation rate: <0.1%.
[0192]Treatment Group 2:
[0193]Brimonidine 0.2%, used off label, has been reported to cause flap dislocation rates of 5-10% and is currently not indicated nor recommended for this purp...
example 3
[0199]0.03% Brimonidine Nasal Spray: 0.9% saline vehicle used and nasal spray administered to patient with nasal congestion. This was repeated for one week without rebound. Complete relief for 3-5 hours was reached per application for treatment of moderate nasal congestion thought to be allergic in nature. Repeat applications×four hours without rebound. Patient population for this test limited to n of 1.
[0200]The proper dose response range can be tested with no more than routine experimentation.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| binding affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com