Systems and methods for production of mixed fatty esters
a technology of fatty esters and mixtures, applied in the direction of transferases, biomass after-treatment, fuels, etc., can solve the problems of increasing the difficulty of acquisition and increasing the limit of the fuel sour
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Production Host Construction
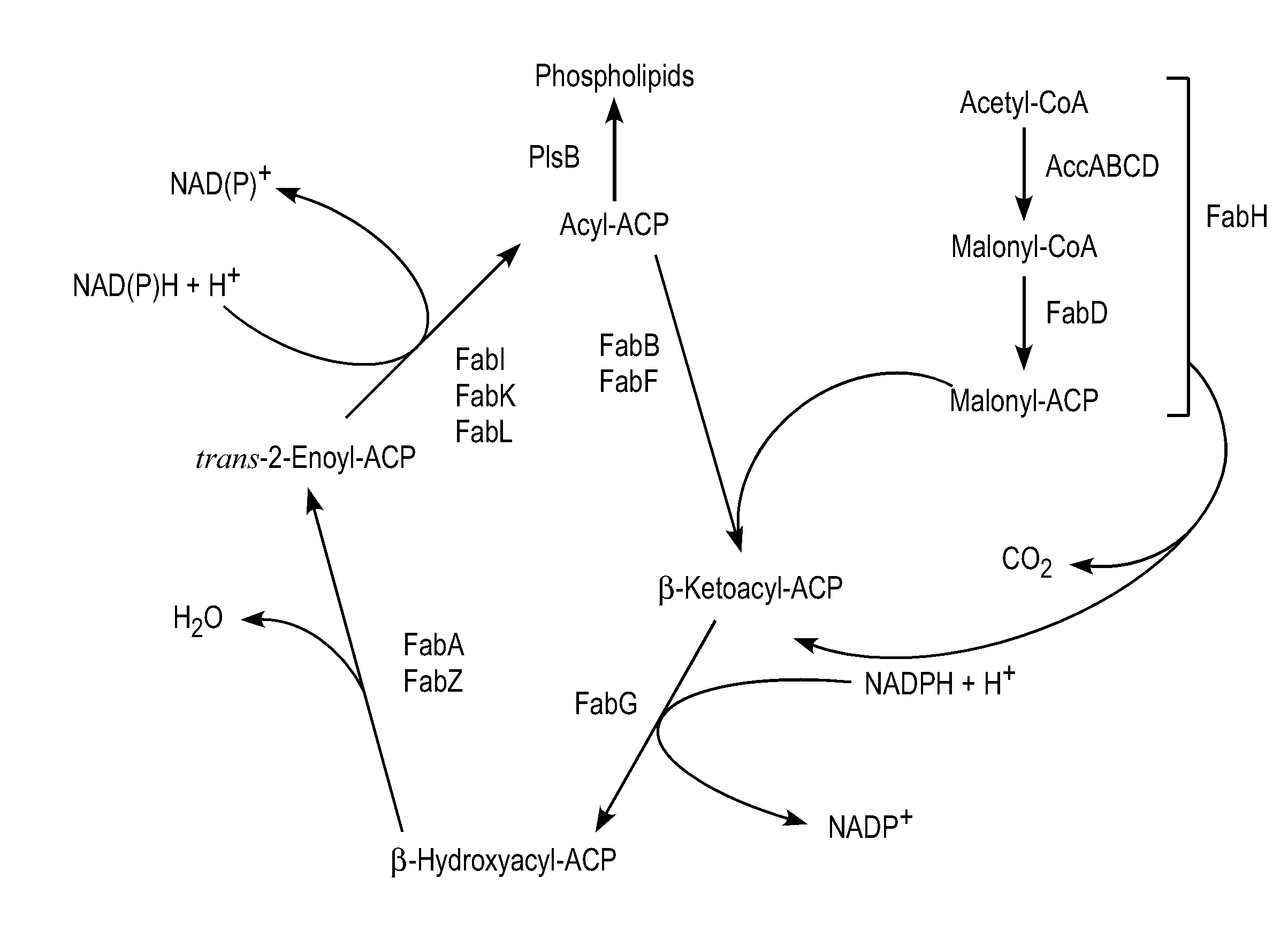
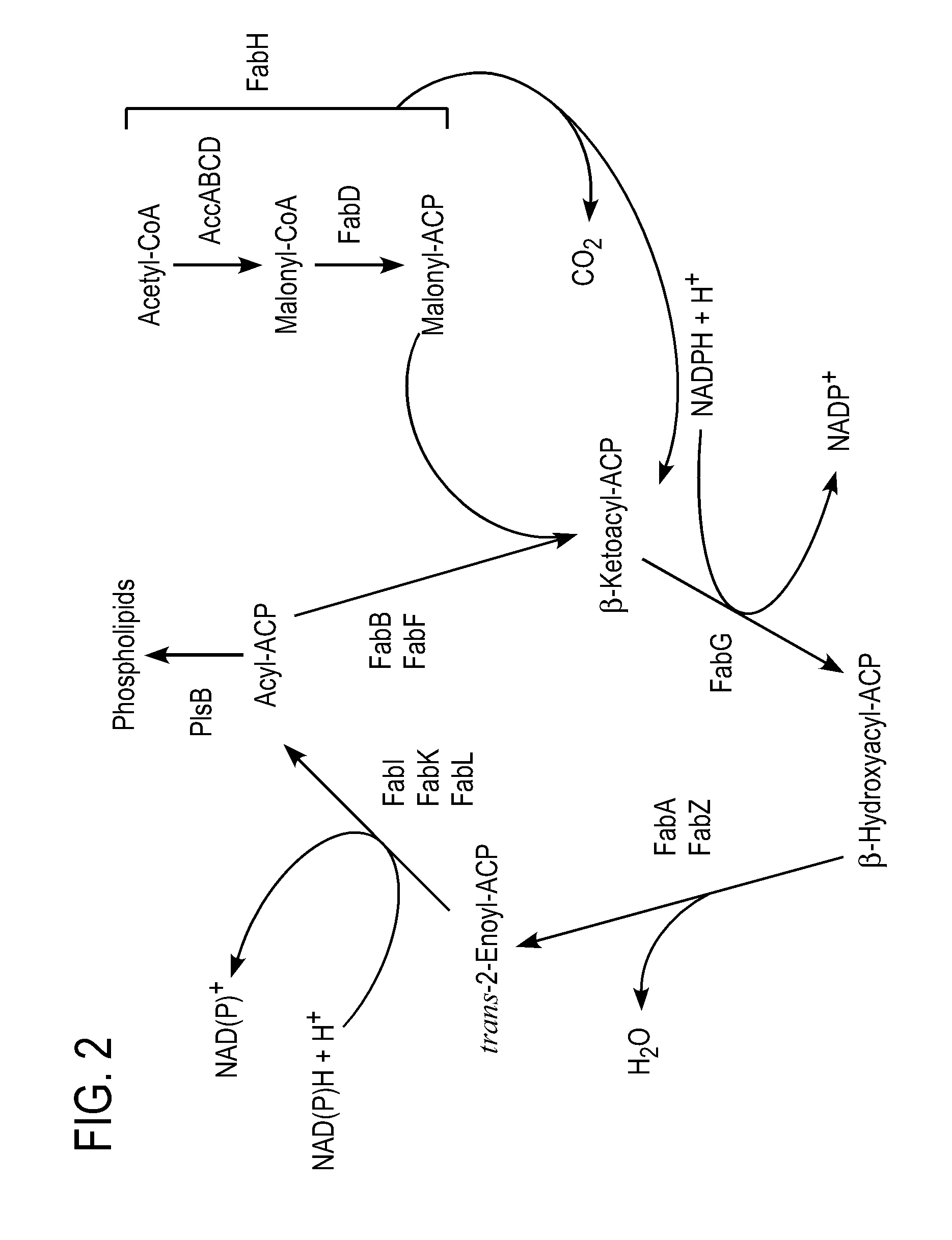
[0492]The present example outlines various production hosts and methods of making them. An exemplary production host is LS9001. LS9001 was produced by modifying C41(DE3) from Overexpress (Saint Beausine, France) to knock-out the fadE gene (acyl-CoA dehydrogenase).
[0493]Briefly, the fadE knock-out strain of E. coli was made using primers YafV_NotI and Ivry_OI to amplify about 830 by upstream of fadE and primers Lpcaf of and LpcaR_Bam to amplify about 960 by downstream of fadE. Overlap PCR was used to create a construct for in-frame deletion of the complete fadE gene. The fadE deletion construct was cloned into the temperature-sensitive plasmid pKOV3, which contained a sacB gene for counterselection, and a chromosomal deletion of fadE was made according to the method of Link et al., J. Bact. 179:6228-6237, 1997. The resulting strain was not capable of degrading fatty acids and fatty acyl-CoAs. This knock-out strain is herein designated as E. coli (DE3, Δfad...
example 2
Additional Production Hosts
[0501]The present example outlines additional modifications that can be made to various production hosts.
[0502]The following plasmids were constructed for the expression of various proteins that are used in the synthesis of fatty acid derivatives. The constructs were made using standard molecular biology methods. The cloned genes were put under the control of IPTG-inducible promoters (e.g., T7, tac, or lac promoters).
[0503]The ‘tesA gene (thioesterase A gene accession NP 415027 without leader sequence (Cho and Cronanu, J. Biol. Chem., 270:4216-9, 1995, EC: 3.1.1.5, 3.1.2.-)) of E. coli was cloned into NdeI / AvrII digested pETDuet-1 (pETDuet-1 described herein is available from Novagen, Madison, Wis.). Genes encoding for FatB-type plant thioesterases (TEs) from Umbellularia californica, Cuphea hookeriana, and Cinnamonum camphorum (accessions: UcFatB1=AAA34215, ChFatB2=AAC49269, ChFatB3=AAC72881, CcFatB=AAC49151) were individually cloned into three different ...
example 3
Medium Chain Fatty Esters
[0508]Alcohol acetyl transferases (AATs, EC 2.3.1.84), which is responsible for acyl acetate production in various plants, can be used to produce medium chain length fatty esters, such as octyl octanoate, decyl octanoate, decyl decanoate, and the like. An AAT gene can be inserted into one of the production hosts described herein by the methods noted in the above examples.
[0509]As will be appreciated by one of skill in the art, fatty esters, synthesized from medium chain alcohol (such as C6 and C8) and medium chain acyl-CoA (or fatty acids, such as C6 and C8) have a relatively low melting point. For example, hexyl hexanoate has a melting point of −55° C. and octyl octanoate has a melting point of −18° C. to −17° C. The low melting points of these compounds make them good candidates for use as biofuels.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com