Combination therapy for the treatment of diabetes and conditions related thereto and for the treatment of conditions ameliorated by increasing a blood glp-1 level
a combination therapy and diabetes technology, applied in the field of diabetes and diabetes treatment, can solve the problems of increasing the incidence of type 2 diabetes worldwide, increasing so as to improve the condition, reduce the risk of diabetes, and improve the effect of blood glucose level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synergistic Effect of GPR119 Agonist and DPP-IV Inhibitor in Lowering an Elevated Blood Glucose Level in Oral Glucose Tolerance Test (oGTT) in Mice
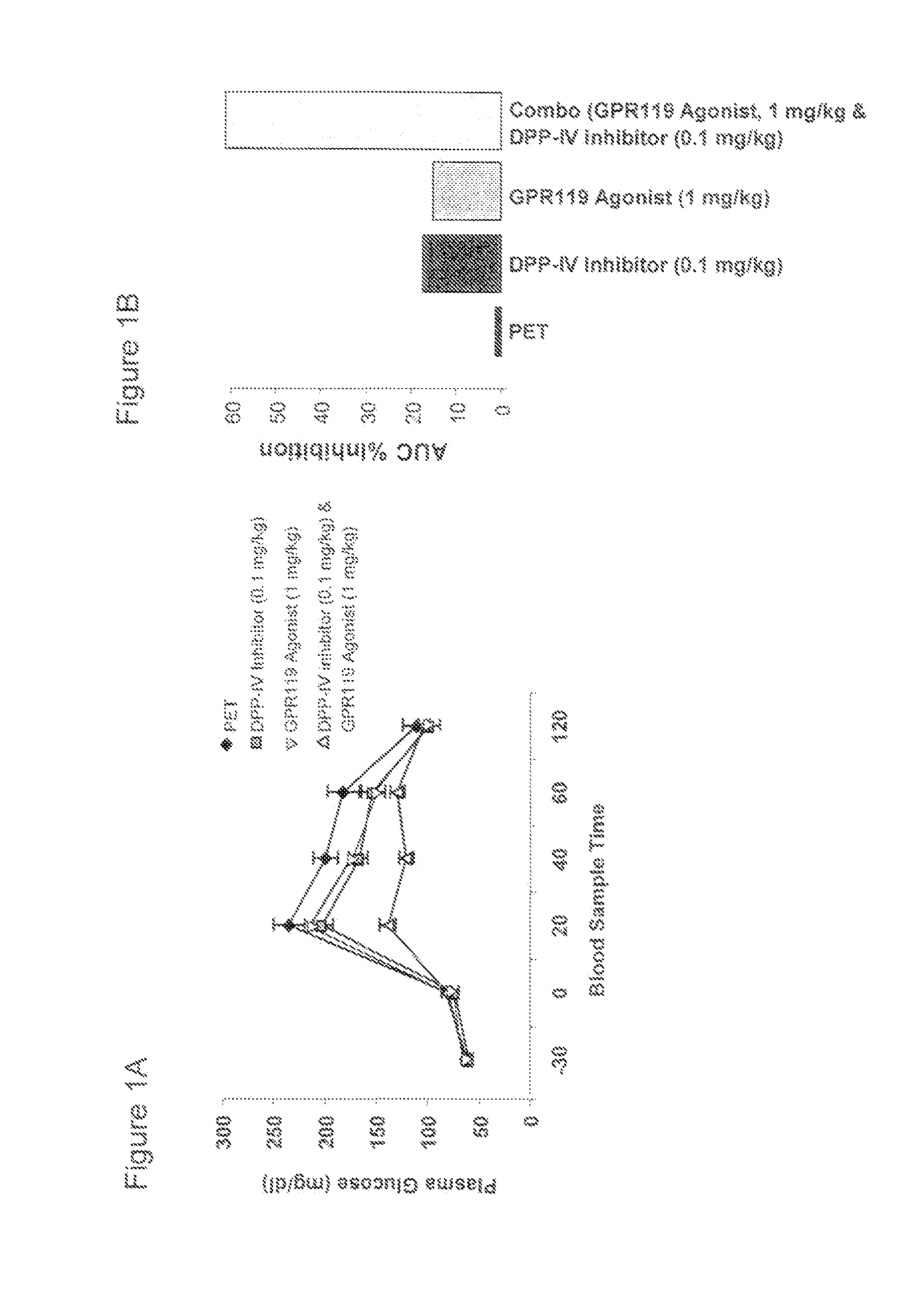
[0655]Oral glucose tolerance test (oGTT) in mice was carried out as described here. Overnight fasted mice (n=6 mice per treatment) were administered via oral gavage with vehicle (PET), a GPR119 agonist (AR231453) at 1 mkg (milligram compound per kilogram of body weight), a DPP-IV inhibitor (AR247810) at 0.1 mkg, or a combination of the GPR119 agonist (1 mkg) and the DPP-IV inhibitor (0.1 mkg). Thirty minutes later, a glucose bolus (3 gram / kg) was then delivered per orally. Plasma glucose levels were determined at the indicated time points over a two hour period using blood (˜5 μl) collected from tail nick and a glucose meter. Glycemic excursion curve was graphed based on data from 6 mice and given in mean values + / −SEM (FIG. 1A). Area Under Curve (AUC) of the glycemic excursion was calculated for each mouse and AUC inhibition (%) was repo...
example 2
Combination of GPR119 Agonist and DPP-IV Inhibitor for Treating or Preventing Diabetes and Conditions Related Thereto
[0658]A GPR119 agonist in accordance with the present invention is selected. A DPP-IV inhibitor in accordance with the present invention is selected.
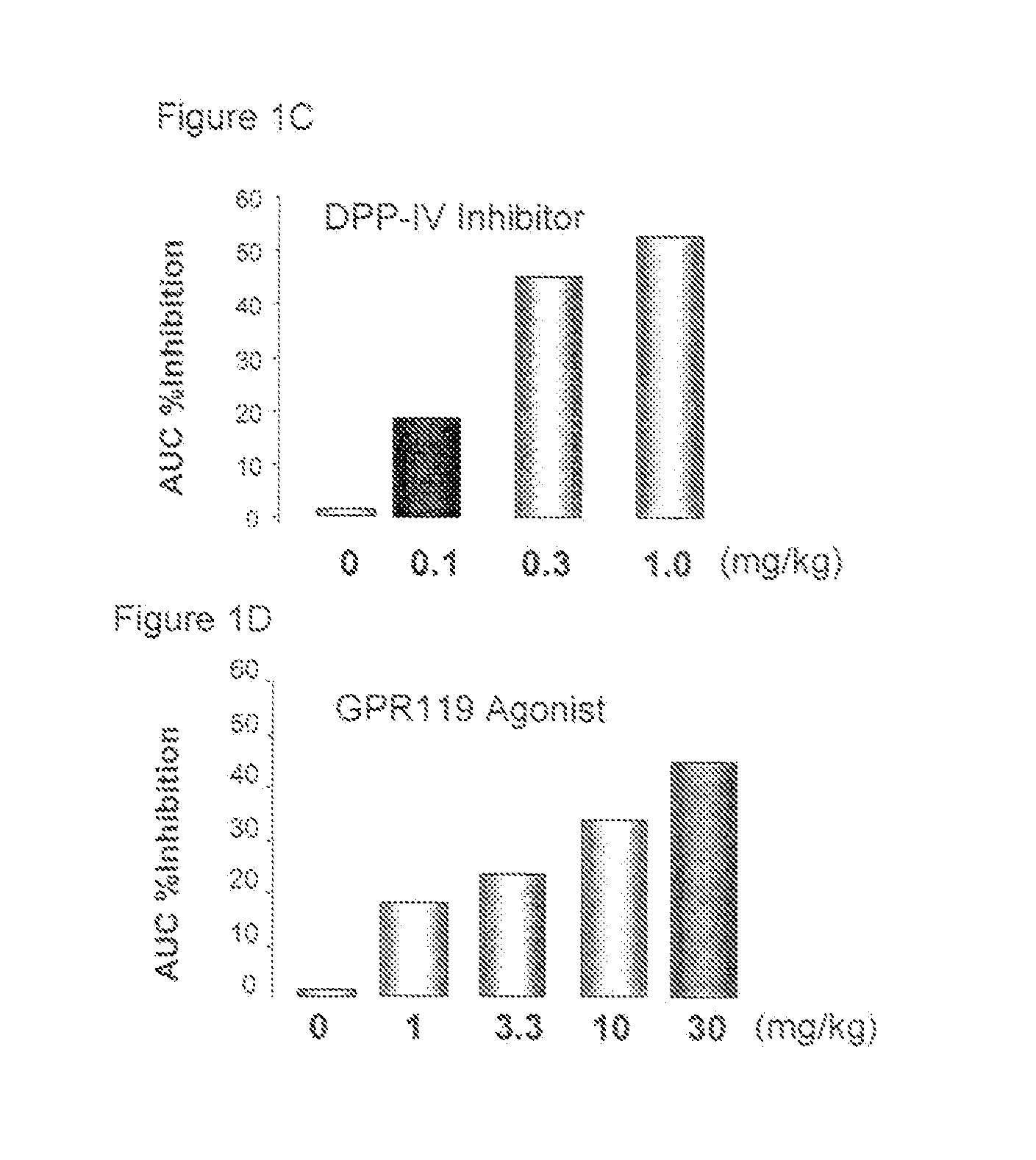
[0659]Titration of the GPR119 agonist with respect to percent inhibition of Area Under Curve (AUC) in mouse oral glucose tolerance test (oGTT) is determined across a dose range from about 0.01 mkg (milligram compound per kilogram of body weight) to about 100 mkg. See Example 1. A dose of the GPR119 agonist producing an AUC inhibition of glycemic excursion of about 15-20% is chosen. Typically, a dose of GPR119 agonist producing an AUC inhibition 30% or less is therapeutically ineffective in this mouse model.
[0660]Titration of the DPP-IV inhibitor with respect to percent inhibition of Area Under Curve (AUC) in mouse oral glucose tolerance test (oGTT) is determined across a dose range from about 0.01 mkg (milligram compound ...
example 3
Synergistic Effect of GPR119 Agonist and DPP-IV Inhibitor in Increasing a Blood GLP-1 Level after Glucose Challenge in Mice
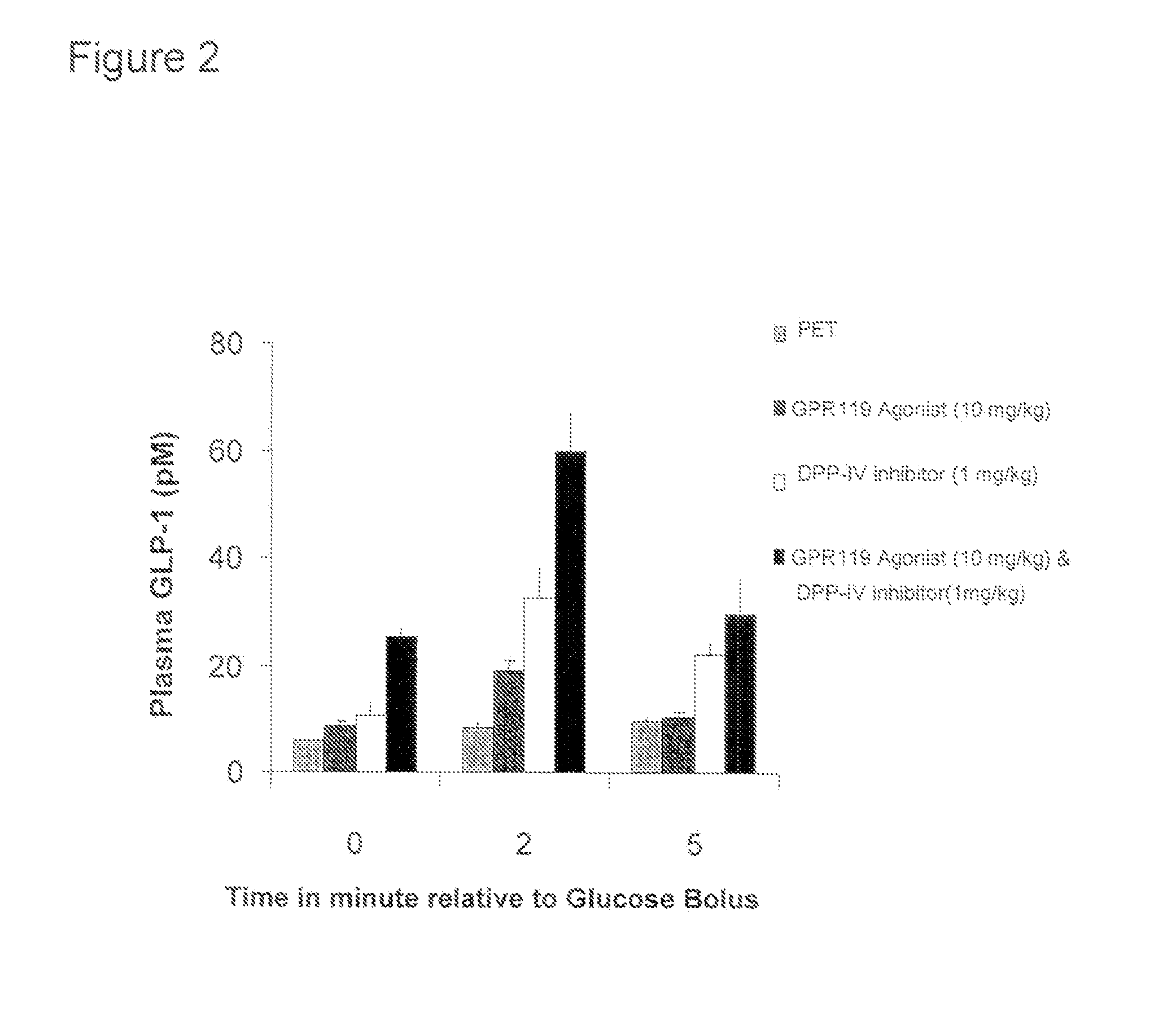
[0664]C57blk / 6 male mice (8 weeks of age) were fasted for 18 hours, and randomly assigned into twelve groups with n=6 for each group. Mice were administered per orally with vehicle (PET), GPR119 agonist (10 mg / kg) DPP-IV inhibitor (1 mg / kg), or a combination of GPR119 agonist and DPP-IV inhibitor, as indicated. The GPR119 agonist (AR231453) and the DPP-IV inhibitor (AR247810) used here are identical to those used in Example 1. Thirty minutes after treatment, a glucose bolus at 3 g / kg were delivered per orally, and plasma were collected at 0 minute (no glucose bolus), and at 2 minutes and 5 minutes after glucose bolus. Plasma GLP-1 levels were determined by using a GLP-1 ELISA kit purchased from Linco Research Laboratory [Glucagon-Like Peptide-1 (Active) ELISA kit, Catalog #EGLP-35K].
[0665]Administration of a GPR119 agonist together with a DPP-IV inhibitor was fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com