Use of tam receptor inhibitors as immunoenhancers and tam activators as immunosuppressors
a technology of tam receptor and inhibitor, which is applied in the field of immunoenhancement or immunosuppression, can solve the problems of compromising the ability to eradicate primary infections, unable to kill pathogens used in traditional vaccines, and immunocompromised people are very vulnerable to opportunistic infections, etc., to suppress the immune response, increase or enhance the activity of tam receptor, and suppress the immune response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Methods
[0316]This Example describes materials and methods that were used in performing Examples 2-9. Although particular methods are described, one of skill in the art will understand that other, similar methods also can be used.
Mice
[0317]C57BL / 6J mice were purchased from The Jackson laboratory, and STAT1 knock-out mice and genetically matched controls were purchased from Taconic. The mutations in the Tyro3− / −, Axl− / − Mer− / − and Ifnar1 mice have been described previously Lu et al., (1999) Nature 398, 723-728; Muller et al. (1994) Science 264, 1918-1921).
Antibodies
[0318]Axl (M-20), IκBβ (C-20), TRAF6, TRAF3, IFNAR1, and IFNAR2 antibodies were obtained from Santa Cruz Biotechnology; phospho-STAT1 Tyr 701, -STAT2 Tyr690, STAT3 Tyr705, STAT1, STAT2, STAT3, IκBα, phospho-P38 Thr180 / Tyr 182, β-actin, and ERK 1 / 2 antibodies were obtained from Cell Signaling Technology; phospho-ERK1 / 2 Thr183 / Tyr185, β-tubulin and ubiquitin antibodies were purchased from Sigma; and p38α and IFN...
example 2
Dendritic Cell Hyper-Activation and Expansion in TAM Triple Knock-Outs
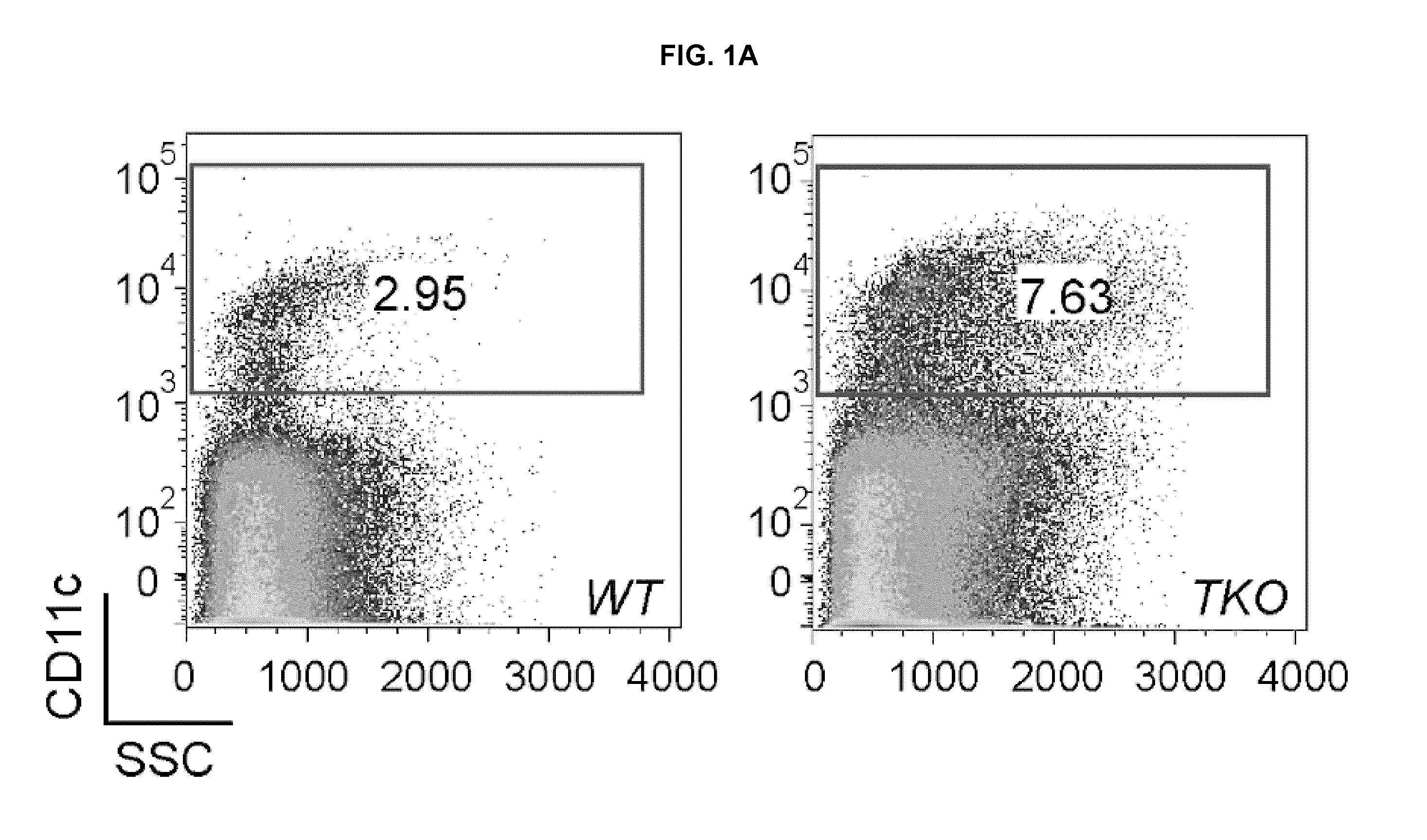
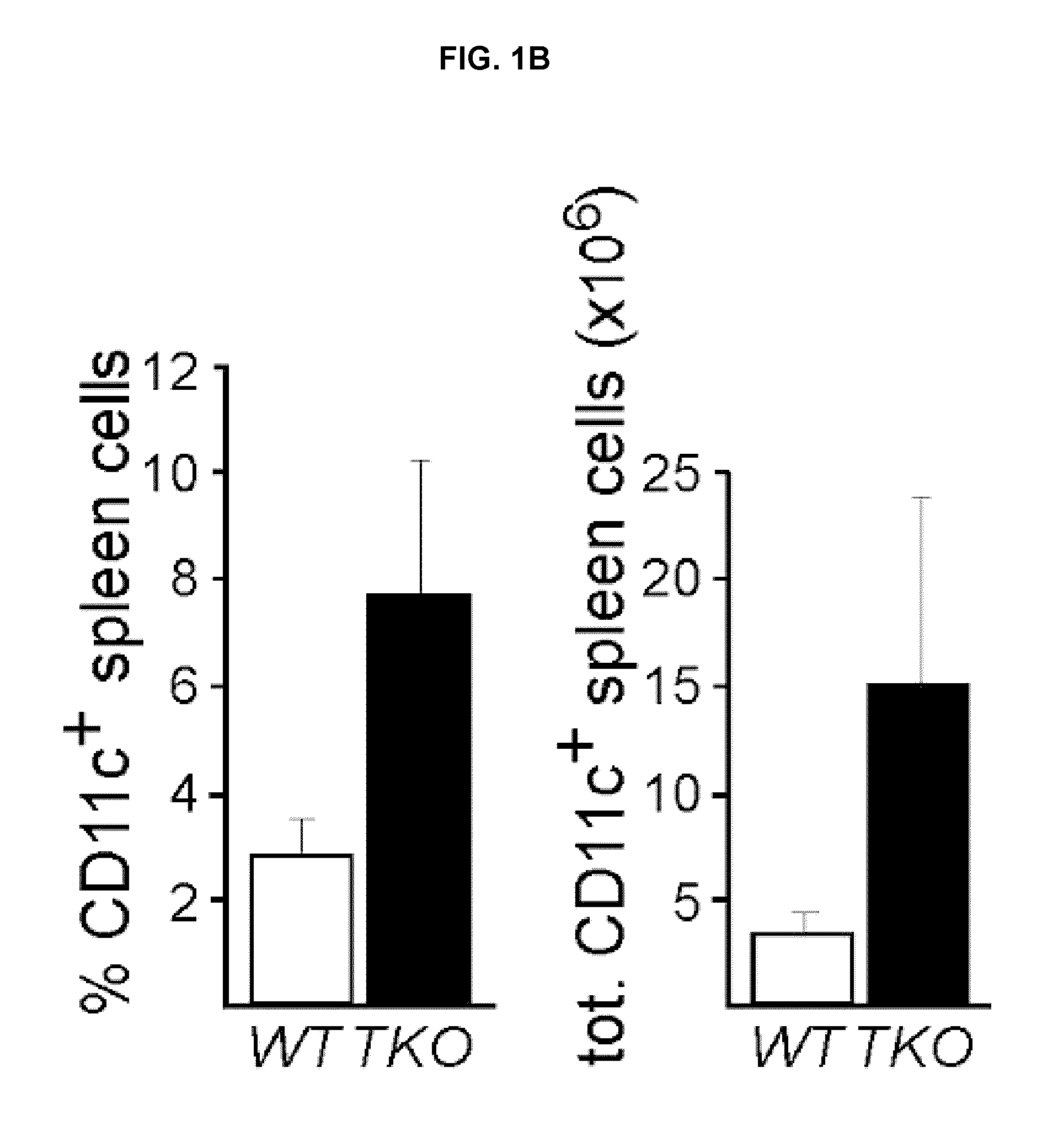
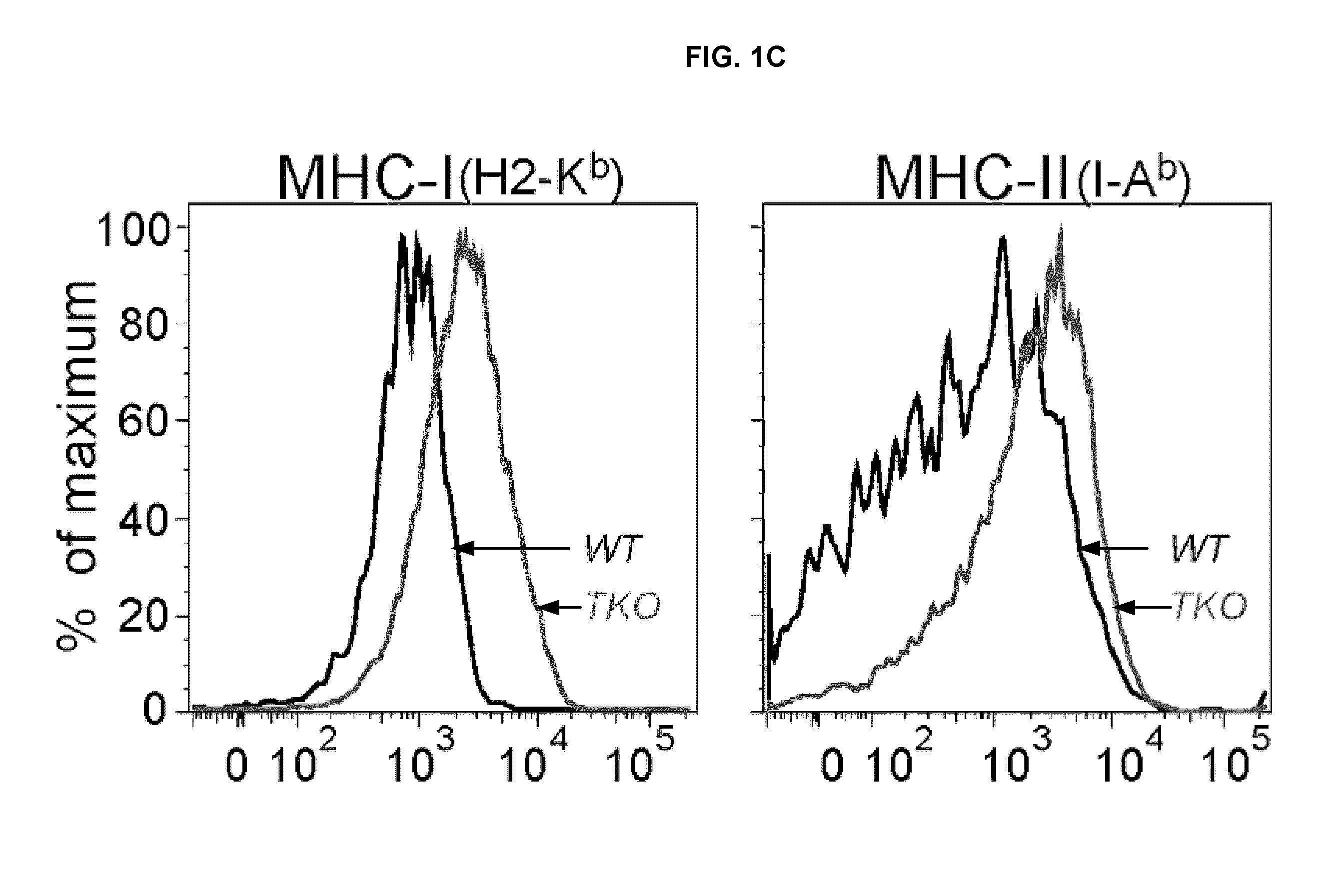
[0329]This Example demonstrates dendritic cell hyper-activation and expansion in TAM triple knock-outs. The immune system phenotypes of TAM TKOs indicated that their autoimmune syndromes might be due to abnormalities in APC physiology (Lu & Lemke (2001) Science 293:306-311). Therefore, the status of the DC subset of professional APCs was assessed in TAM TKO spleens. The percentage of splenic CD11c+ cells was markedly elevated in TAM TKOs relative to wild-type (WT), as was the absolute number of CD11c+ cells (FIGS. 1A, 1B). Splenic DCs were also hyperactivated in the triple mutants. CD11c+ DCs in TAM TKOs expressed higher levels of MHC class I and class II (FIG. 1C), and BAFF / BlyS (FIG. 1D), which is elevated in patients with autoimmune diseases such as SLE (Collins et al., (2006) Arthritis Res Ther 8:R6).
[0330]TAM TKO CD11c+ DCs express super-elevated levels of MHC class II and B7.2 in response to intraperitoneal ...
example 3
TAM Receptor Activation Inhibits TLR-Induced Cytokine Production in DCs
[0331]This Example demonstrates that TAM receptor agonists inhibit TLR-driven cytokine production in WT DCs. Given the results described in Example 2, it was determined whether treatment with TAM receptor agonists might inhibit TLR-driven cytokine production in WT DCs. Analyses were performed in both splenic CD11c+ DCs, and in DCs differentiated from mouse bone marrow (BM) in the presence of FLT-3 ligand (Gilliet et al., (2002) J Exp Med 195:953-958).
[0332]In both DC populations, the major TAM receptors expressed were Axl and Mer (FIG. 1G), with no detectable Tyro 3. The TAM receptors are activated by the binding of two closely-related, soluble ligands—Gas6 and Protein (ProS; Prasad et al., (2006) Mol Cell Neurosci 33: 96-108; Stitt et al., (1995) Cell 80: 661-670). Overnight (15 hours) co-incubation of recombinant Gas6 with CpG substantially inhibited TLR9-induced production of Type I IFNs, IL6, and TNFα in wild...
PUM
| Property | Measurement | Unit |
|---|---|---|
| systolic blood pressure | aaaaa | aaaaa |
| systolic blood pressure | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com