Composition for inhibition of transplant rejection containing the cordyceps mycellia extract as an active ingredient
a technology of cordyceps and mycellia, which is applied in the direction of drug compositions, immunological disorders, antibody medical ingredients, etc., can solve the problems of side effects of this drug, and the use of vegetable worm extracts as immunosuppressants has not been disclosed in the prior period, so as to suppress the production of antibodies and arrest the oozing of sores
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
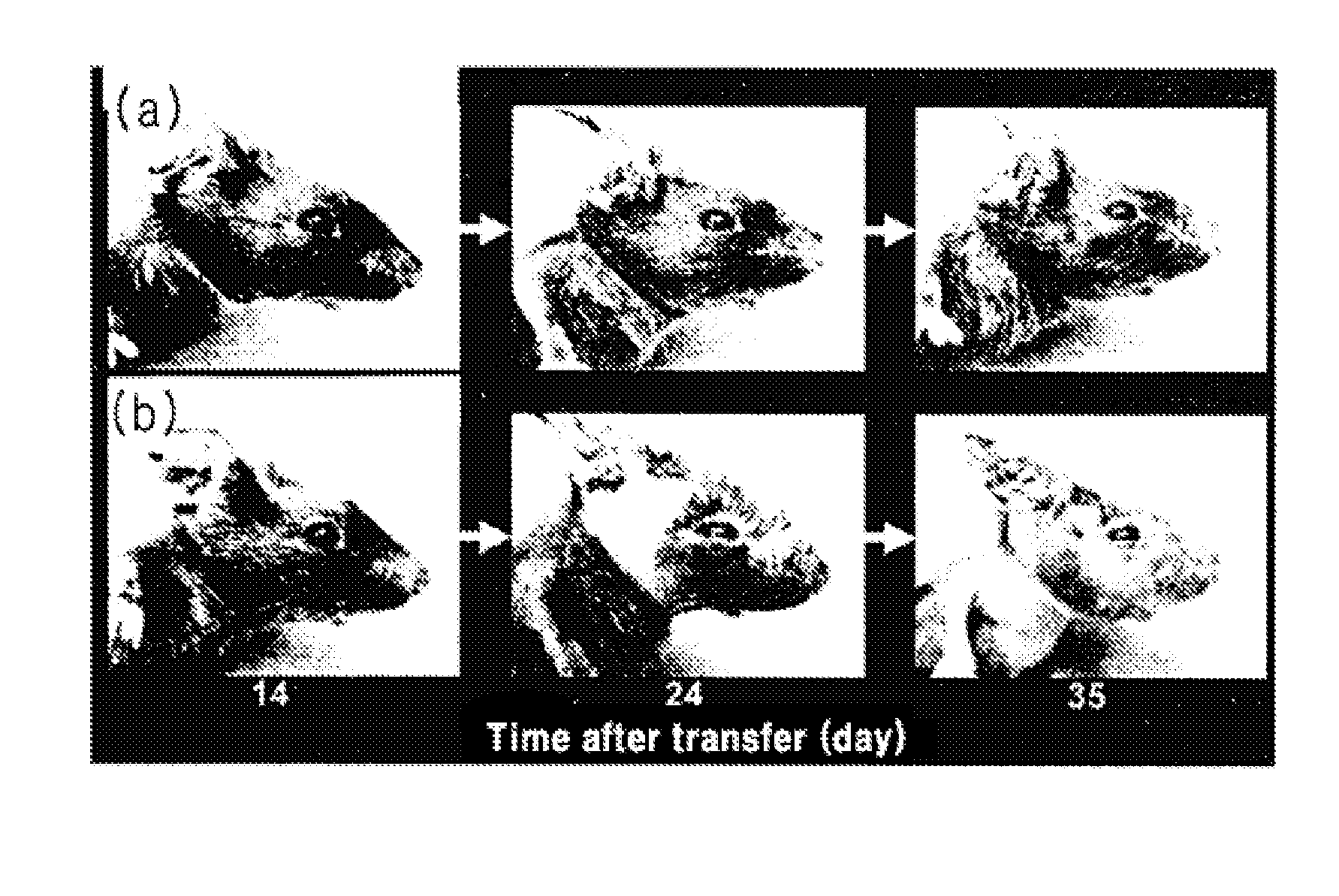
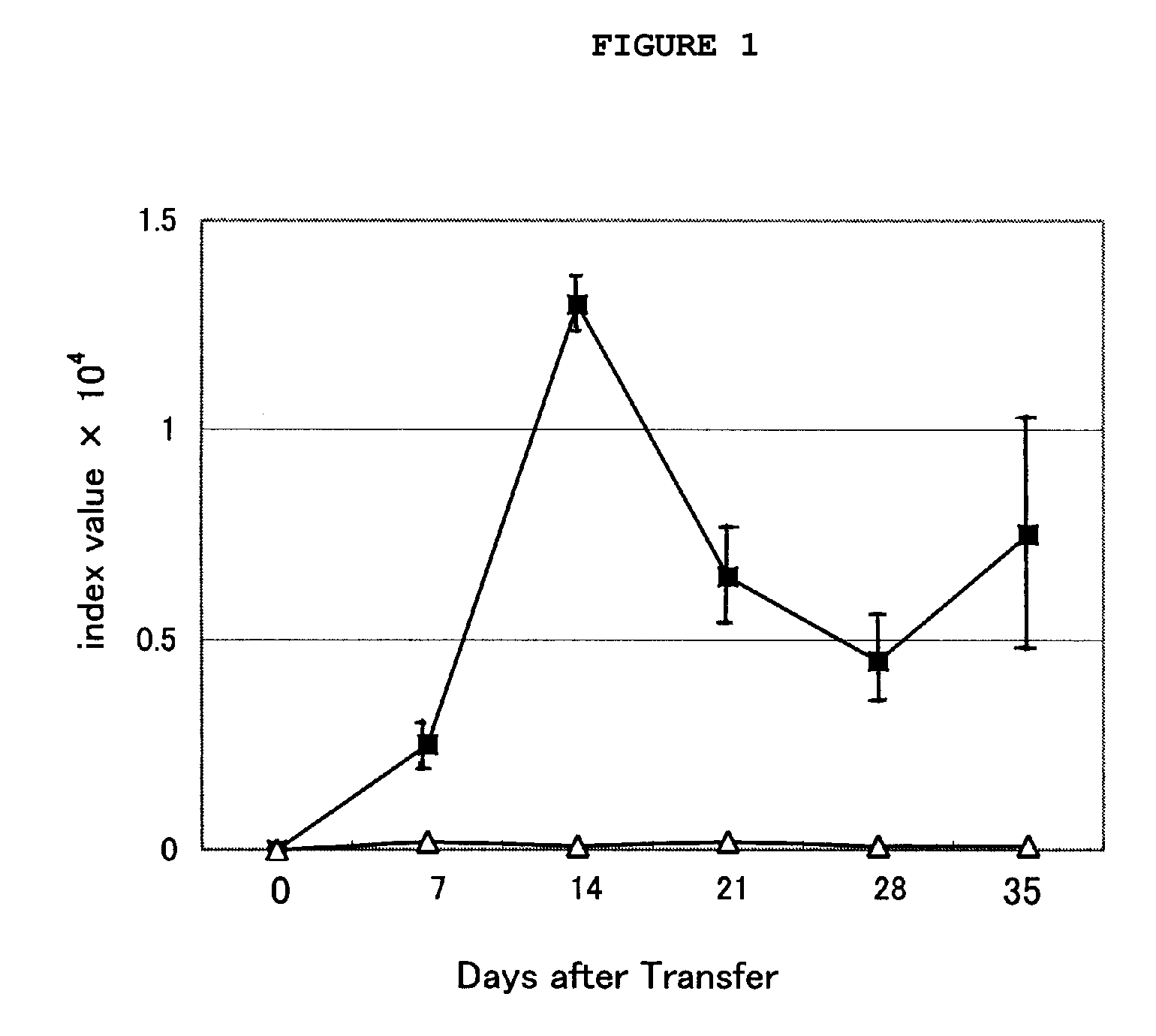
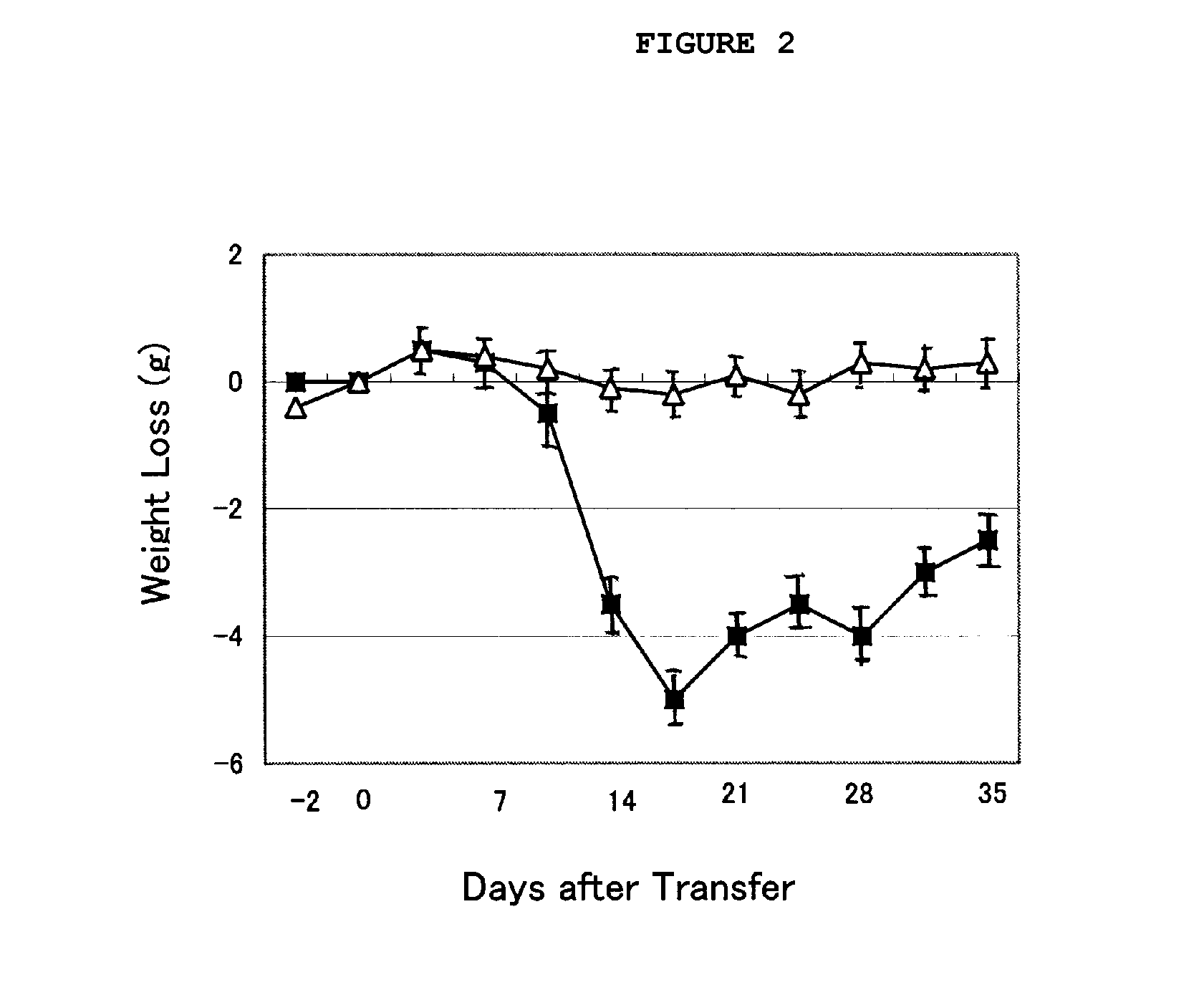
Immunosuppression Assay on Pemphigus Mouse Model
[0039]An immunosuppression test was conducted with the cordyceps mycellia extract of the present invention on pemphigus mouse models, as follows.
[0040]Two S129 Dsg3− / − mice and 24 S129 Rag2− / − mice were prepared as transplantation donors and recipients, respectively. The recipient mice were divided into four groups of six: control (CMC administered), comparative group (cyclophosphamide (CPA) administered), experimental group 1 and experimental group 2 (cordyceps mycellia extract (Cordyma®; Han Kook Sin Yak) administered). The transplantation donor was subjected to an immune reaction by administering a Dsg3-His protein thereto, as described below. First, 10 μg of mouse Dsg3-His protein was emulsified with the same amount of complete Freund's adjuvant (CFA) and subcutaneously injected into the mice (1st). One week after the first immunization, an emulsion of 10 μg of mouse Dsg3-His protein in the same amount of incomplete Freund's adjuva...
preparation example 1
Preparation of Pharmaceutical Formulations
[0051]1-1. Preparation of Powder
Cordyceps mycellia extract 1 gDextrin0.1 g
[0052]The above ingredients were mixed and loaded into an airtight sac to produce powder.
[0053]1-2. Preparation of Tablet
Cordyceps mycellia extract500 mgDextrin 45 mgMg Stearate 5 mg
[0054]These ingredients were mixed and prepared into tablets using a typical tabletting method.
[0055]1-3. Preparation of Capsule
Cordyceps mycellia extract500 mgDextrin 50 mg
[0056]These ingredients were mixed and loaded into gelatin capsules according to a typical method to produce capsules.
[0057]1-4. Preparation of Injection
Cordyceps mycellia extract50mg / mlDiluted HCl BPadded to form pH 3.5NaCl BP injectionup to 1ml
[0058]The cordyceps mycellia extract was dissolved in a suitable volume of an NaCl BP injection, and the solution was adjusted to a pH of 3.5 with diluted HCl BP and to a desired volume with NaCl BP injection, followed by sufficient mixing. The solution was loaded into transpare...
PUM
| Property | Measurement | Unit |
|---|---|---|
| total volume | aaaaa | aaaaa |
| total volume | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com