Homeopathic complex
a technology of complex and homeopathy, applied in the field of homeopathic complex, can solve the problems of inability to reconstitute a homeopathic mother tincture from a homeopathic mother tincture, the loss of chemical toxicity, and the inability to treat the disease with conventional treatment, so as to achieve the effect of treating or prophylaxis of infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0419]Subject—Springer Spaniel Female neutered, age 5 years old on Day 0.
Condition / Symptoms of Subject
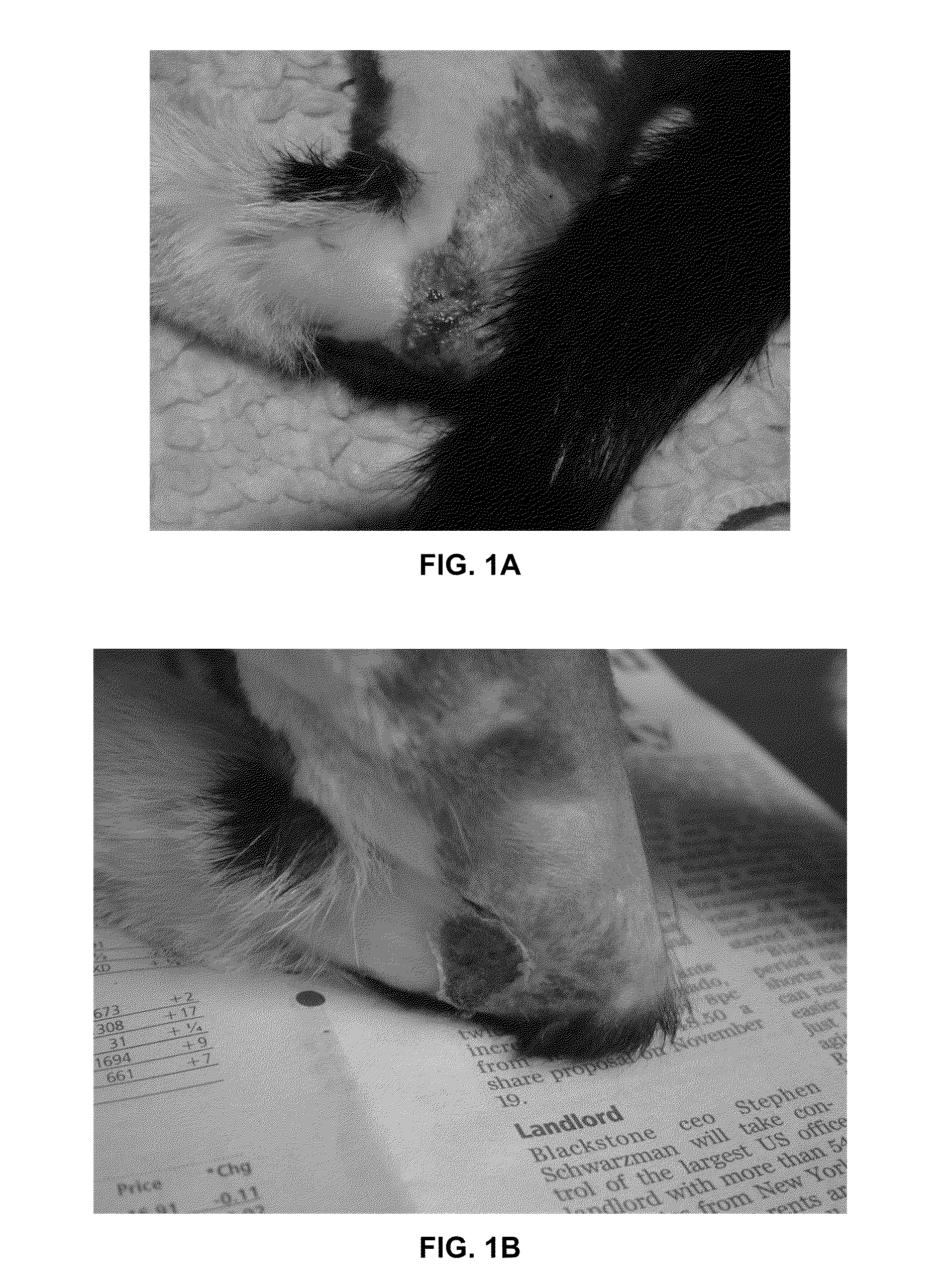
[0420]The patient presented with non-healing wound of what appeared to be a MRSA-type infection resulting from minor surgical wound as a result of elective surgery in the area of the stopper pad. The owner described the original wound as having been completely closed after surgery.[0421]The patient had an ulcerative skin lesion which had started after minor surgery and had gradually extended.[0422]There had been no response to repeatedly changed conventional antibiotics (Fuciderm®) or the antibiotic impregnated dressings used[0423]After using a number of conventional antibacterial therapeutics the owner had decided to try an alternative approach.
Treatment
[0424]On Day 0 the patient was taken off all oral conventional medication. A cream consisting of Core A and Core B with the addition Fucidin was applied by mixing a tube of Topical Ophthalmic Antibiotic FUCITHALMIC® Viscous Eye prop...
example 2
[0428]Subject—Feline male neutered of indeterminate age
Conditions / Symptoms of Subject
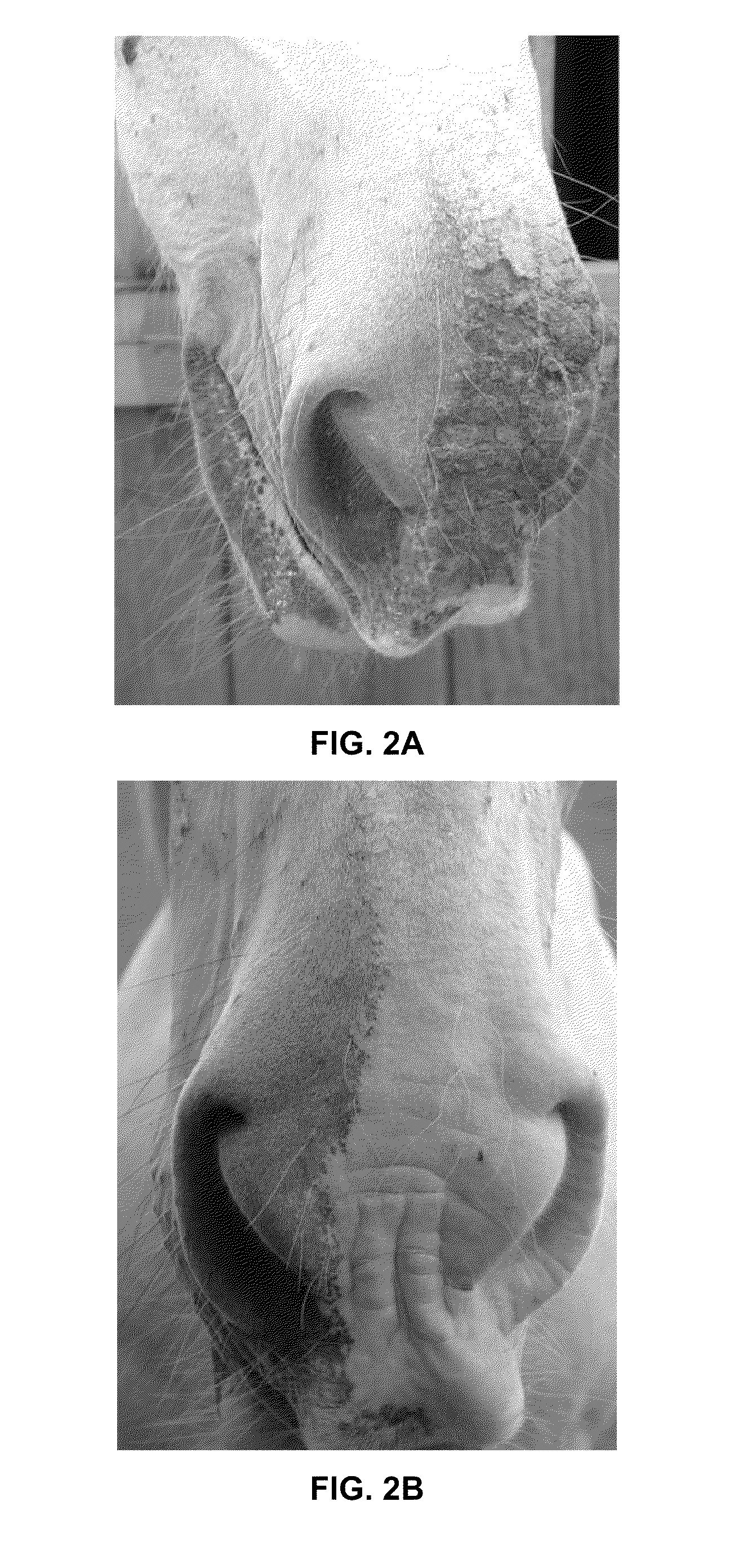
[0429]The subject originally presented as a post road traffic accident patient, with multiple traumatic skin injuries and multiple fractures of the left femur the latter were repaired by open reduction and intramedullary pining. The fracture site had to be partially re-broken as there was already cartilaginous callus repair with very mal-aligned bone fragments.
[0430]The subject was post shock. It was already in the healing phase of injury, but the wounds were still open and fresh. The bone fractures were attempting to heal out of alignment. The subject was initially unable to walk on the hind legs despite having one un-fractured leg. The musculature bones and wounds were all painful. The wounds were due to grazing probably from contact with a road surface and so friction burns as well as wounds, bruising and fractures were present.
Treatment
[0431]The fractures were treated by open reduction. Immediat...
example 3 (
Example 3(b)
[0444]Subject—Herd of male and female pigs less than 1 year of age
Conditions / Symptoms of Subject
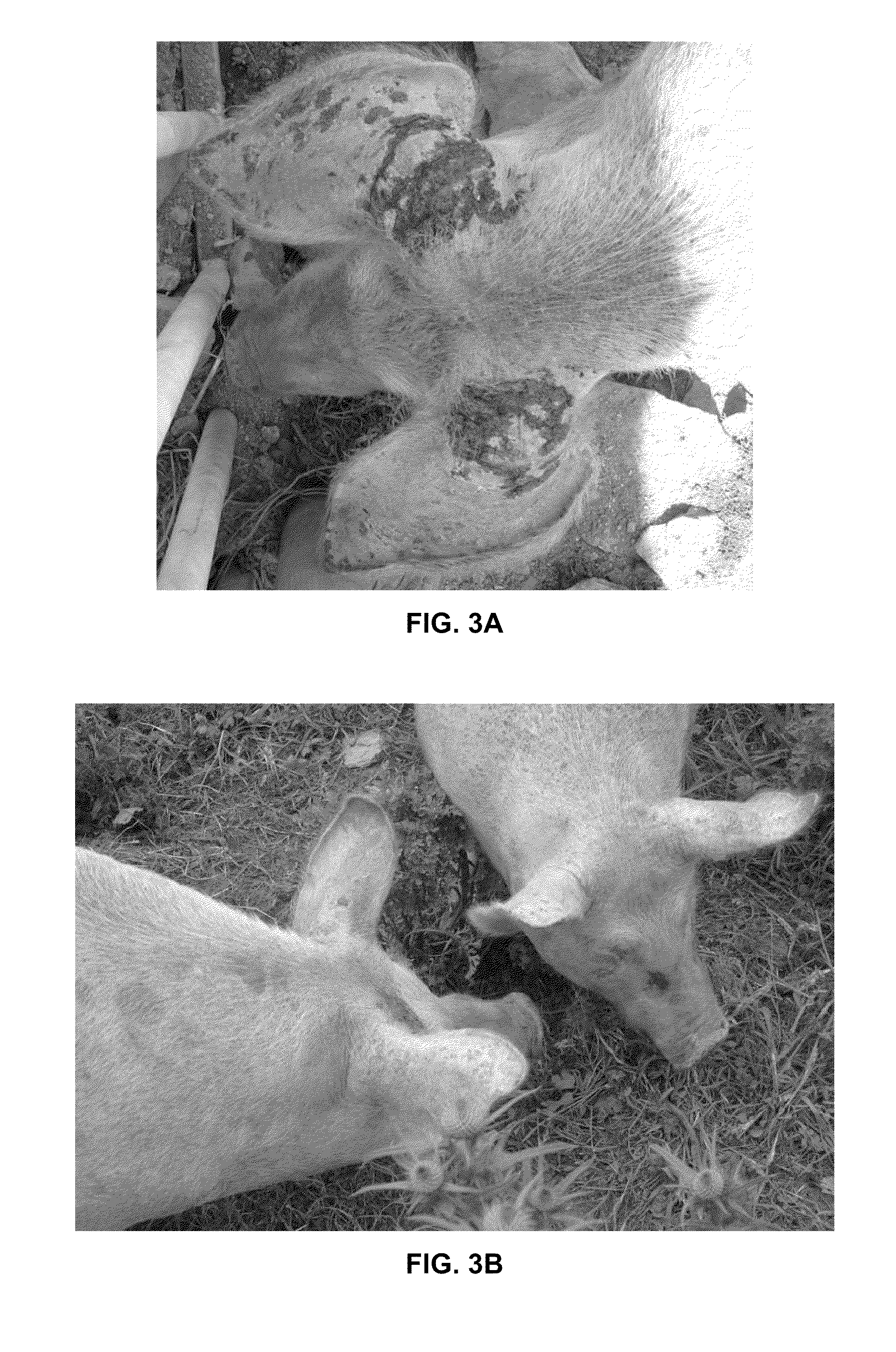
[0445]Subjects were presented with acute severe photo-dermatitis after being left out on new pasture during a period of unbroken sunshine. The subjects presented much as a case of bad human sunburn would (FIG. 3a) with the whole patient having a bright red burning hot inflamed tender skin with crusting of the skin at the worst affected areas where there was deep cracking due to third degree burning. There were areas of peeling and scabbing of the affected skin.
Treatment
[0446]Due to success with previous cases in a range of other species a treatment with Core A and Core B combined was used. However, due to the number of animals a novel delivery method was required. Aloe Vera Veterinary Spray (Forever Living Products) was used as a carrier for Core A and Core B. Once daily spraying of the carrier with Core A and Core B was decided upon with a second spray when possible at feedin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| body weight | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com