Methods for measuring the insulin receptor alpha subunit
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0172]Hereinafter, the present invention will be described more specifically with reference to Examples.
1. Construction of Human Insulin Receptor α-Subunit cDNA
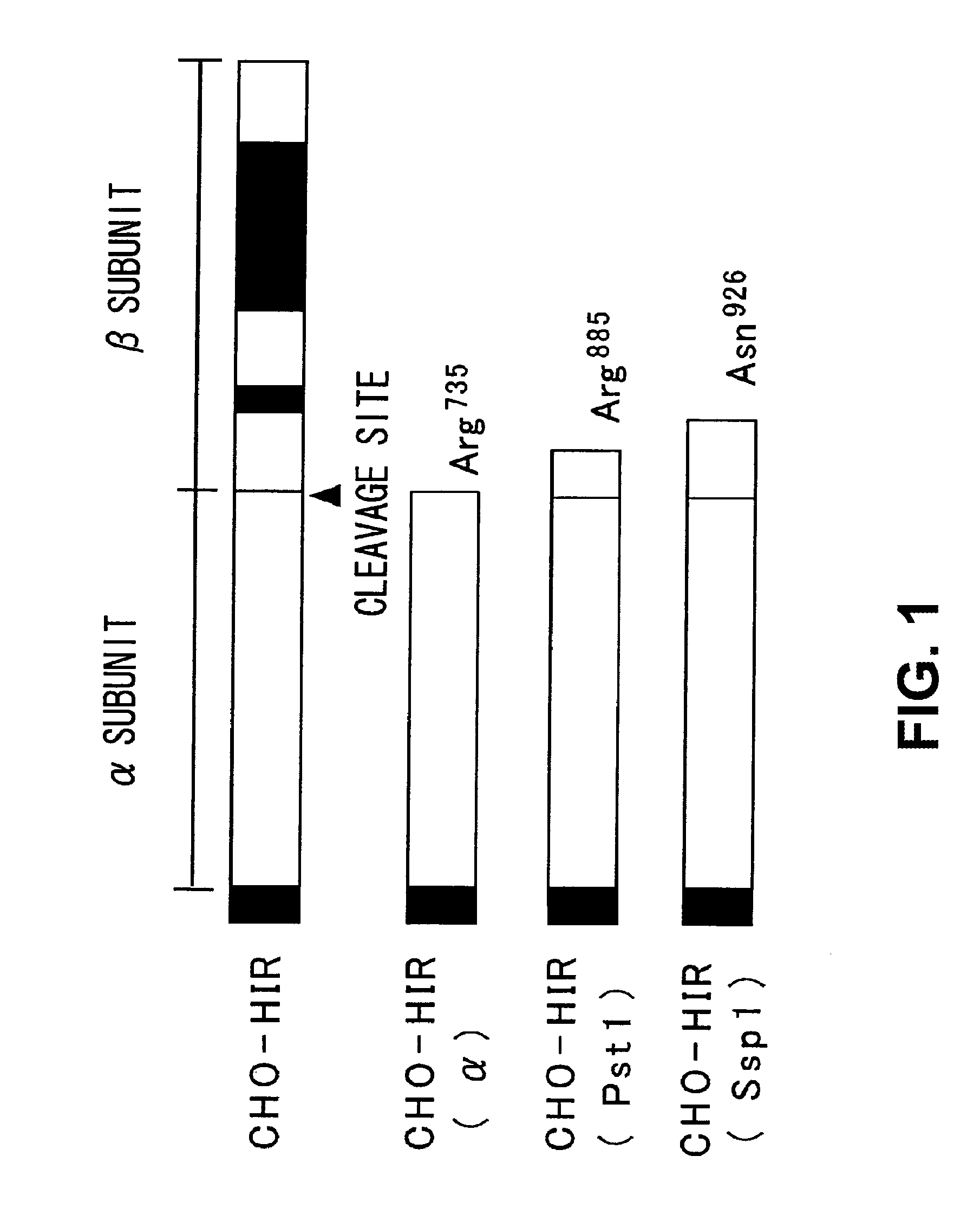
[0173]cDNA for causing secretion of insulin receptor α-subunit into the culture supernatant from CHO cells were constructed. The structures of the cDNAs constructed in the present example are shown in FIG. 1. Each of the cDNAs was obtained by using pcDL1-HIR717 (Ebina et al. Cell. 40, 747-758, 1985) comprising the full-length cDNA (NM—000208) of the human insulin receptor and digesting it at different positions. Each of the cDNAs has the following structures, respectively.
CHO-HIR: insulin receptor α-subunit+β-subunit
CHO-HIR(α): insulin receptor α-subunit alone
CHO-HIR(PstI): α-subunit and amino acids 1 to 150 in the N terminal side of the β-subunit
CHO-HIR(SspI): α-subunit and amino acids 1 to 191 in the N terminal side of the β-subunit
[0174]The β-subunit transmembrane domain is in positions 195 to 217 from the N-terminal side....
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| absorbance at wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com