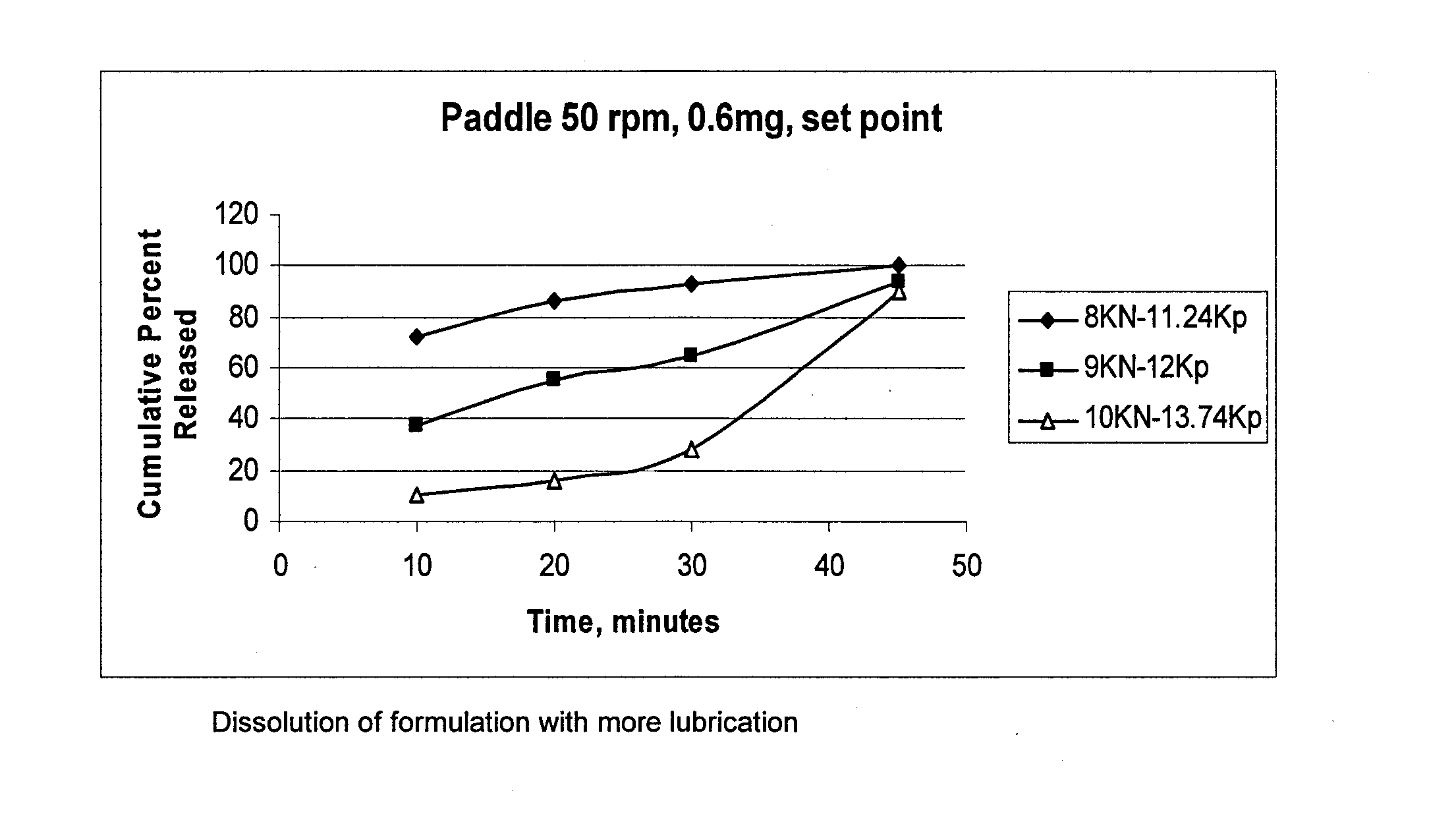
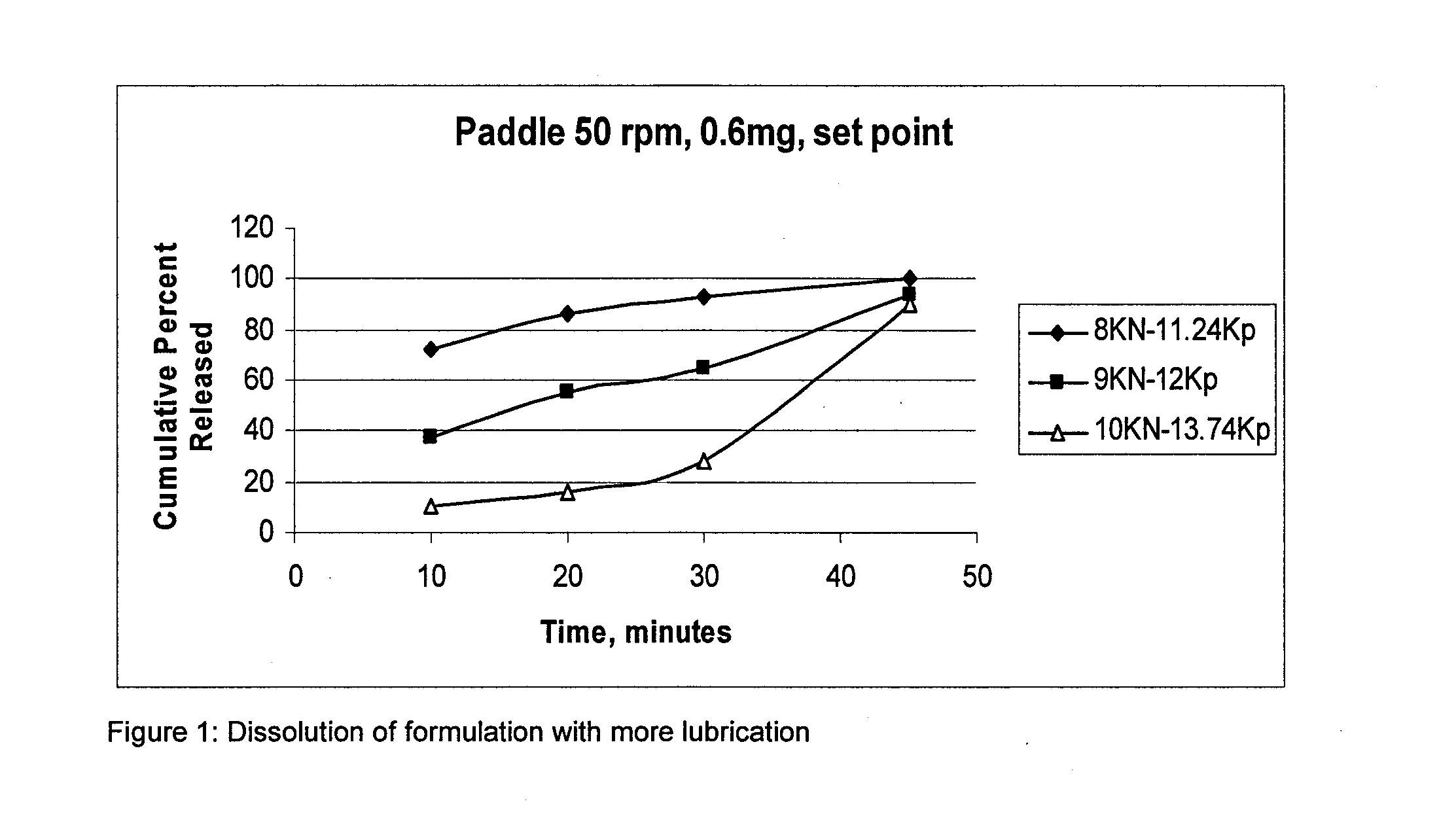
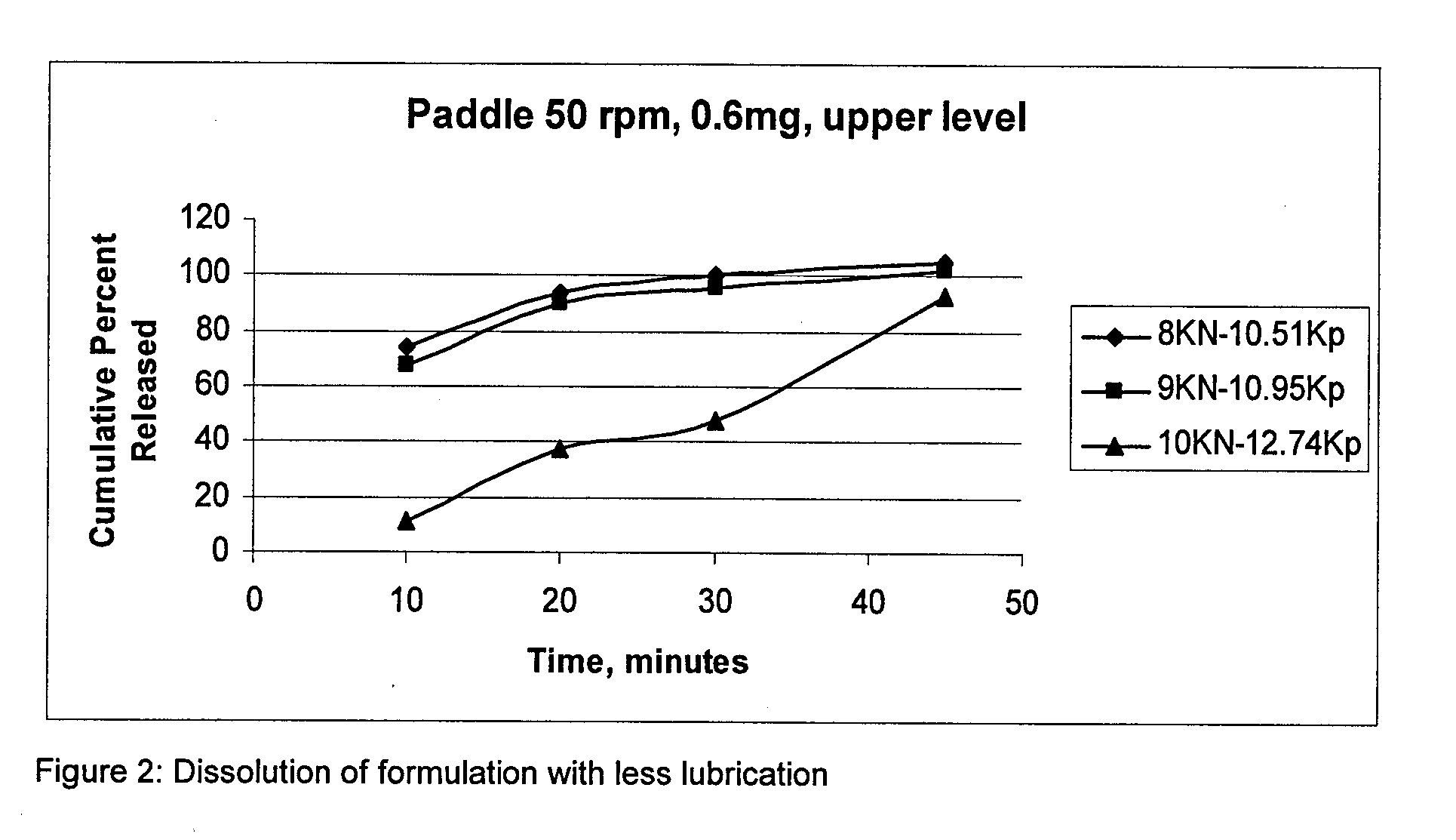
Pharmaceutical composition
a technology of pharmaceutical compositions and compositions, applied in the field of pharmaceutical compositions, can solve problems such as insufficient protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Pharmaceutical Composition 1
[0243]
IngredientAmount (mg)PercentSalmon calcitonin0.80.16Micronized 5-CNAC22845.6Avicel PH 102(E)24147.94Crospovidone, NF255Magnesium stearate50.3Total500
[0244]Salmon calcitonin, 5-CNAC and crospovidone were blended together in a first blending step. Avicel PH 102 was screened and added to the mixture and blended in a second blending step. Magnesium stearate was then added and the mixture was blended further in a final blending step. The final blend was compressed into a 500 mg tablet and evaluated in a Rhesus monkey. The results are shown in FIG. 5.
example 2
Alternative Pharmaceutical Composition (3 BATCHES)
[0245]The same composition as in Example 1 was made, i.e. a composition comprising:
IngredientAmount (mg)PercentSalmon calcitonin0.80.16Micronized 5-CNAC22845.6Avicel PH 102(E)24147.94Crospovidone, NF255Magnesium stearate50.3
[0246]However, in contrast to Example 1 Salmon calcitonin and Avicel PH 102 were blended in a first blending step. 5-CNAC and crospovidone were then added to the first blend in a second blending step. Finally, Magnesium Stearate was added in a final blending step.
[0247]The final blend was then compressed at 3 different compression levels to obtain 3 different batches of tablets each having a different hardness, in order to provide 3 different disintegration times:
[0248](i) 1 min 10 secs DT
[0249](ii) 5 mins 40 secs DT
[0250](iii) 8 mins 51 secs DT
example 3
Alternative Pharmaceutical Composition
[0251]A similar blend was made to that of Example 1, except that an amount of Cab-o-sil was added to form a composition comprising:
IngredientAmount (mg)PercentSalmon calcitonin0.60.12Micronized 5-CNAC22845.6Avicel PH 102(E)24147.94Crospovidone, NF255Cab-o-sil1.50.3Magnesium stearate51Total500
[0252]Salmon calcitonin, 5-CNAC and crospovidone were blended in a first blending step. Avicel and Cab-o-sil were screened and added in a second blending step. Finally, Magnesium stearate was added in a final blending step. The final blend was compressed into a 500 mg tablet. The incorporation of Cab-o-sil improved the compression profile of the tablet.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com