HIV-1 Peptides, Nucleic Acids, and Compositions and Uses Thereof
a technology of immunodeficiency virus and peptide, which is applied in the field of human immunodeficiency virus 1 (hiv1) polypeptides, can solve the problems of limited number of known neutralization targets that are insensitive to such masking, poor immunogenicity, and limited progress towards a hiv-1 vaccine capable of inducing a protective humoral response. to achieve the effect of inhibiting the infection of a cell
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Methods
[0096]Monoclonal Antibodies (mAbs)
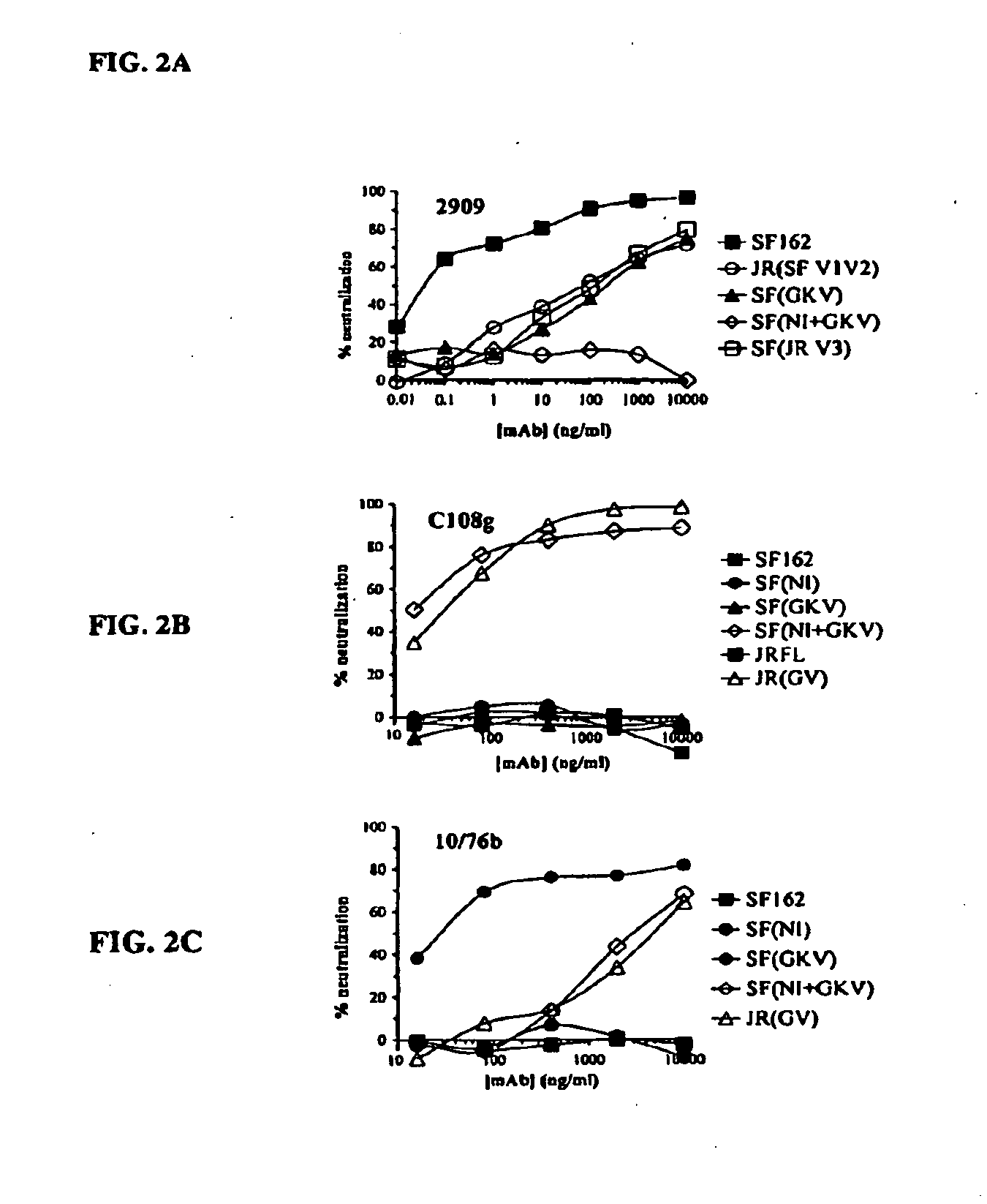
[0097]The monoclonal antibodies in these examples were obtained as follows. C108g was isolated from a chimpanzee infected with the IIIB strain of HIV-1 and subsequently immunized with isolated MN gp120 (Warrier et al. (1994) J. Virol. 68(7):4636-4642). The characteristics of C108g and its epitope were previously described (Warrier et al. (1994) J. Virol. 68(7):4636-4642; Vijh-Warrier et al. (1995) Mol. Immunol. 32:1081-1092; Wu et al. (1995) J. Virol. 69(4):2271-2278; Pinter et al. (2005) J. Virol. 79(11):6909-6917). 10 / 76b was isolated from a rat immunized with soluble HXB10 gp120 (McKeating et al. (1993) J. Virol. 67(8):4932-4944) and characteristics of its epitope have been described (Shotton et al. (1995) J. Virol. 69:222-230). 2909 was isolated from an HIV-1-infected human subject by screening for neutralization of HIV-1 pseudotyped with SF162 Env (Gorny et al. (2005) J. Virol. 79(8):5232-5237).
Chimeric and Variant Forms of...
example 2
Mapping the Critical Determinants of 2909 Reactivity to the V2 Domain
[0100]The V2 and V3 domain determinants required for 2909 mAb binding were mapped by examining the neutralizing activity of this mAb against a series of SF162 mutants and variants bearing changes in both the of these domains. As previously reported, 2909 possessed extremely potent neutralizing activity for SF162, with an ND50 in the low pg / ml range (0.000058 μg / ml). Substituting the V3 domain of SF162 with that of the clade B consensus sequence (identical to that of the JR-FL isolate) resulted in a significant attenuation in potency, with an almost 900-fold increase in ND50. The SF162 sequence differs from that of the clade B consensus at three positions, substitution of T for H at the highly polymorphic position 13, A for T at position 22 and D for Eat position 25. An analysis of the effect of single residue substitution at these three positions on attenuation of neutralization activity of 2909 showed that the thr...
example 3
Mapping the V2 Determinants of 2909 Reactivity
[0103]To map determinants in the V2 domain required for 2909 reactivity, the neutralizing activity of 2909 for a series of V1 / V2 mutants and variants was examined. These included a chimeric Env protein in which the V1 / V2 domain of SF162 Env were replaced by that of JR-FL Env (which is not recognized by 2909), and V2 domain mutants used to introduce the C108g and 10 / 76b epitopes into SF162 Env (FIG. 1). Substitution of the complete SF162 V1N2 domain by the JR-FL sequence resulted in complete loss of reactivity, confirming a critical role for the V1 / V2 domain in the 2909 epitope (Table 2). Consistent with this, substitution of the SF162 V1 / V2 domain into JR-FL Env resulted in neutralization sensitivity similar to that of the SF162 chimera with the JR-FL V3 domain, indicating that all of the determinants for the selective reactivity of 2909 for SF162 over JR-FL were localized to the V1 / V2 and V3 domains. Analysis of V2 mutants that were use...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| mean diameter | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com