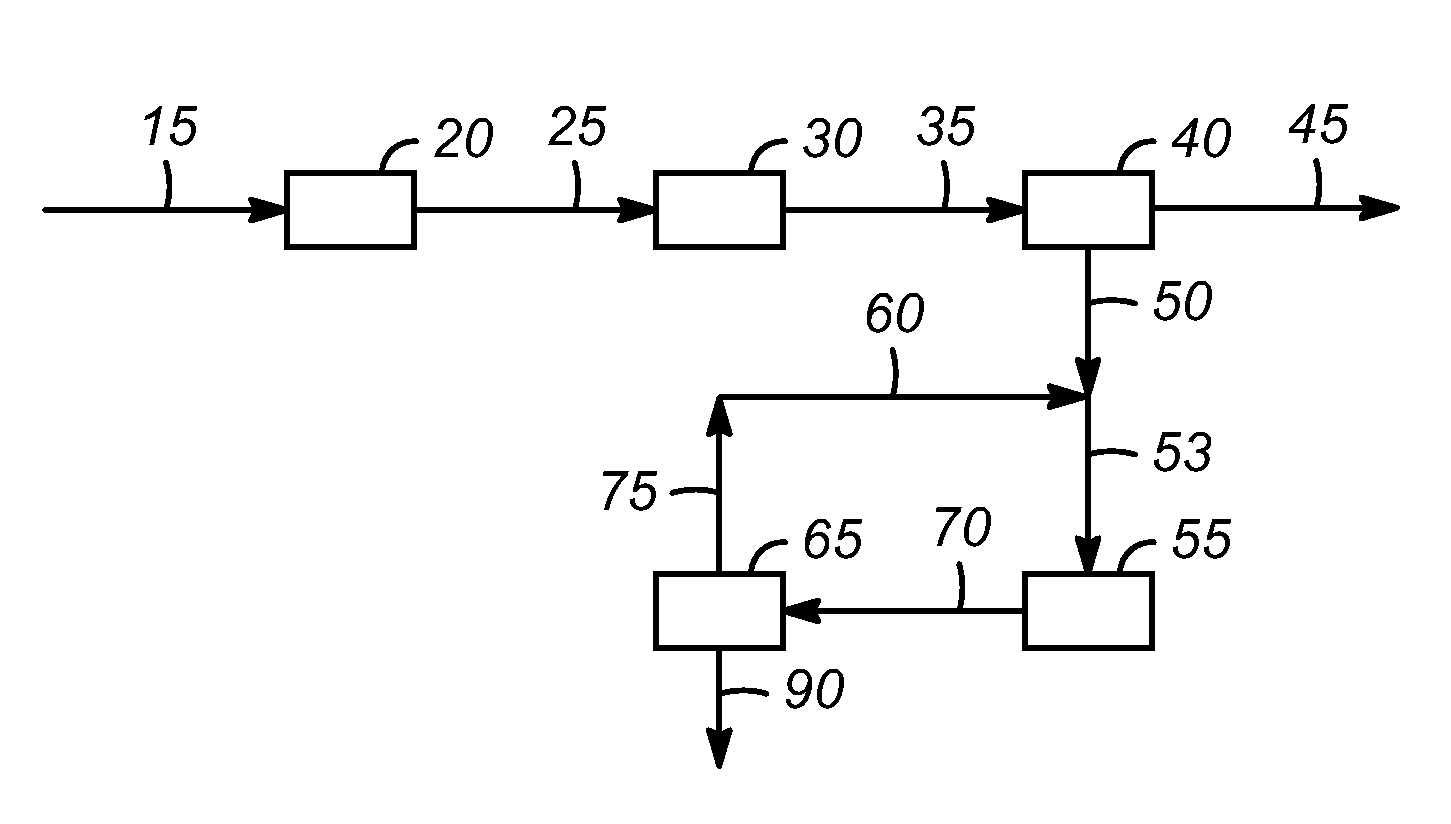
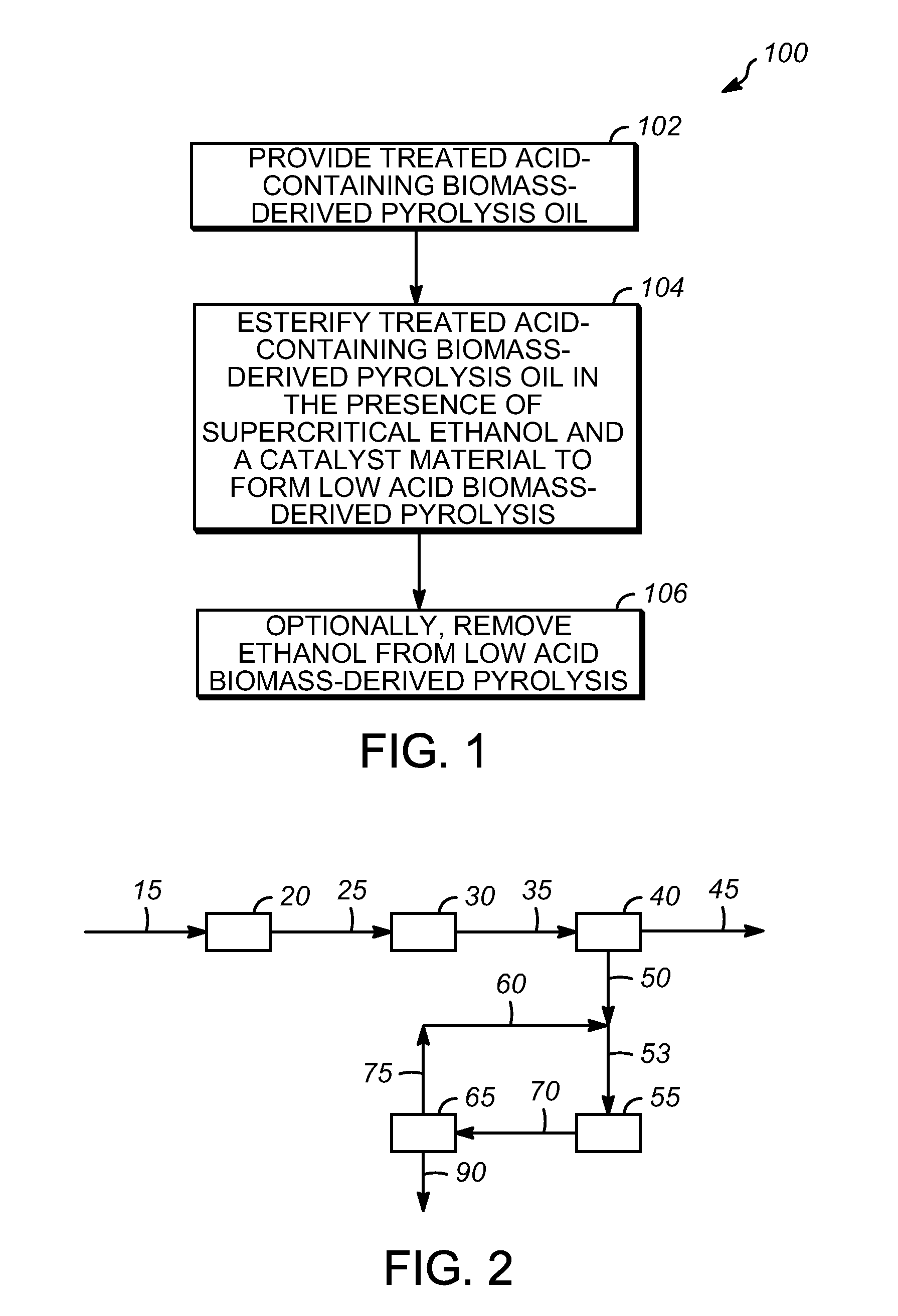
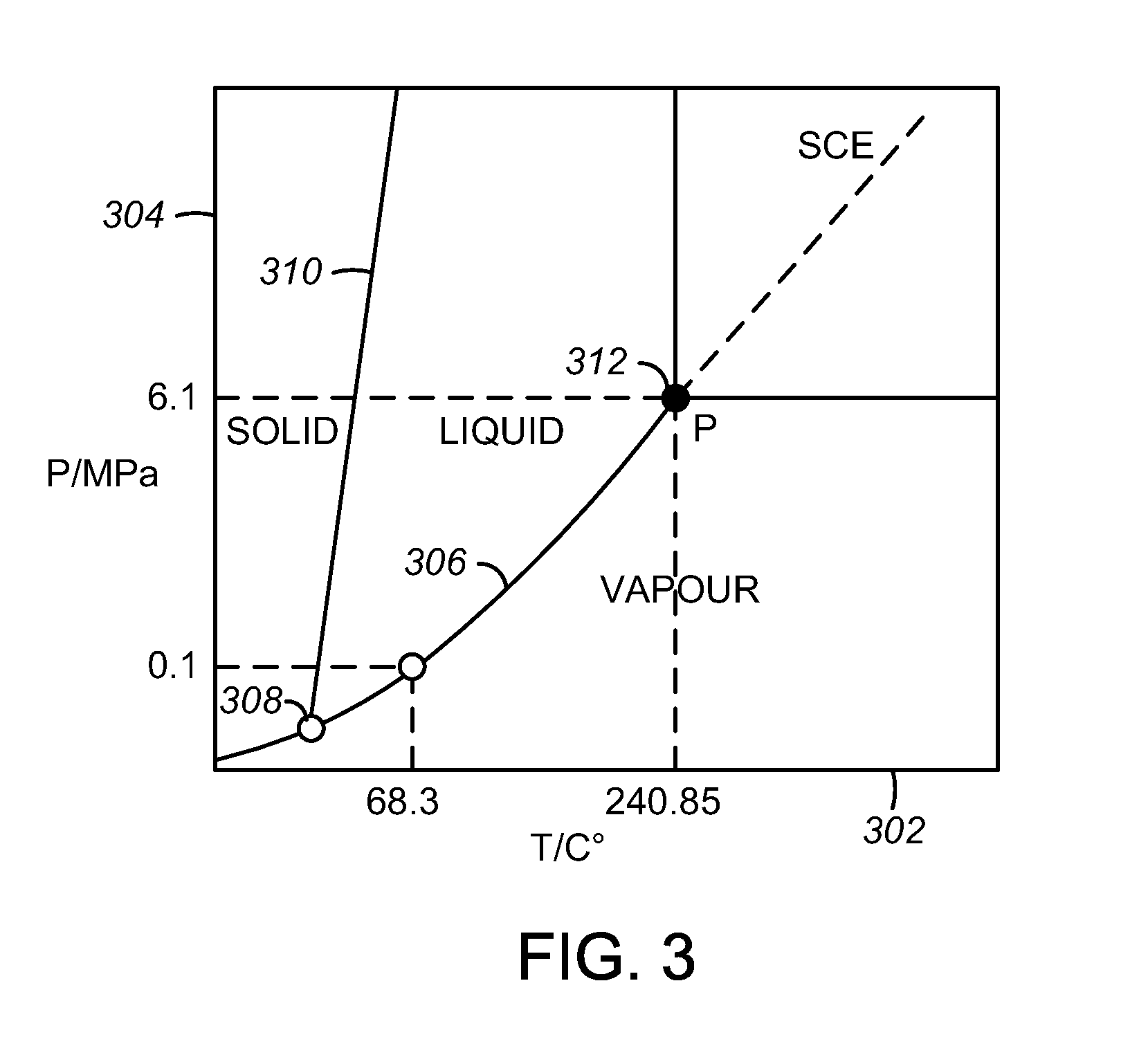
Processes for producing low acid biomass-derived pyrolysis oils
a biomass-derived pyrolysis oil, low acid technology, applied in the direction of biofeedstock, liquid carbonaceous fuel, petroleum industry, etc., can solve the problems of limiting the utilization of biomass-derived pyrolysis oil as a biofuel, low energy density, corrosive, etc., and achieves low energy density and poor thermal stability of biomass-derived pyrolysis oil. , the effect of reducing the number of pyrolysis oil oxidation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0053]A pre-blended feed was prepared for use in several experiments, as will be discussed in further detail below. The pre-blended feed included about 50 weight percent (wt %) ethanol and about 50 wt % low water biomass-derived pyrolysis oil. The blended feed had a total acid number (TAN) of about 110.5, where 40.9 of the TAN was attributed to carboxylic acid and about 69.6 of the TAN was attributed to phenolic.
example 2
[0054]One hundred (100) grams of the pre-blended feed was added to an autoclave including 5.0 grams of 0.5% platinum on γ alumina. A head pressure of nitrogen about 2.75 MPa (400 psig) was added to the autoclave. The autoclave was then heated to a temperature in a range of about 255° C. The temperature was maintained for about 1.8 hours, and a maximum pressure of about 12.41 MPa (1800 psig) was achieved to provide supercritical conditions for the ethanol and to esterify the oil in the pre-blended feed. After the reaction, the pressure at room temperature was 4.13 MPa (600 psig). No solids were found in the autoclave, and about 10 grams of the liquid was converted to gas. The TAN of the esterified feed was about 65.62, where 25.24 of the TAN was attributed to carboxylic acid and 40.38 of the TAN was attributed to phenolic.
example 3
[0055]Ninety (90) grams of the pre-blended feed was added to an autoclave including 4.5 grams of about 70% to about 85% silicon oxide and about 15% to about 30% aluminum oxide. A head pressure of nitrogen in a range of about 2.75 MPa (400 psig) was added to the autoclave. The autoclave was then heated to a temperature in a range of about 255° C. The temperature was maintained for about 1.8 hours, and a maximum pressure of about 1800 psig was achieved to provide supercritical conditions for the ethanol and to esterify the oil in the pre-blended feed. After the reaction, the pressure at room temperature was 3.79 MPa (550 psig). No solids were found in the autoclave, and about 6% of the liquid was converted to gas. The TAN of the esterified feed was about 64.5, where 22.43 of the TAN was attributed to carboxylic acid and 42.06 of the TAN was attributed to phenolic.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pressure | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| residence time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com