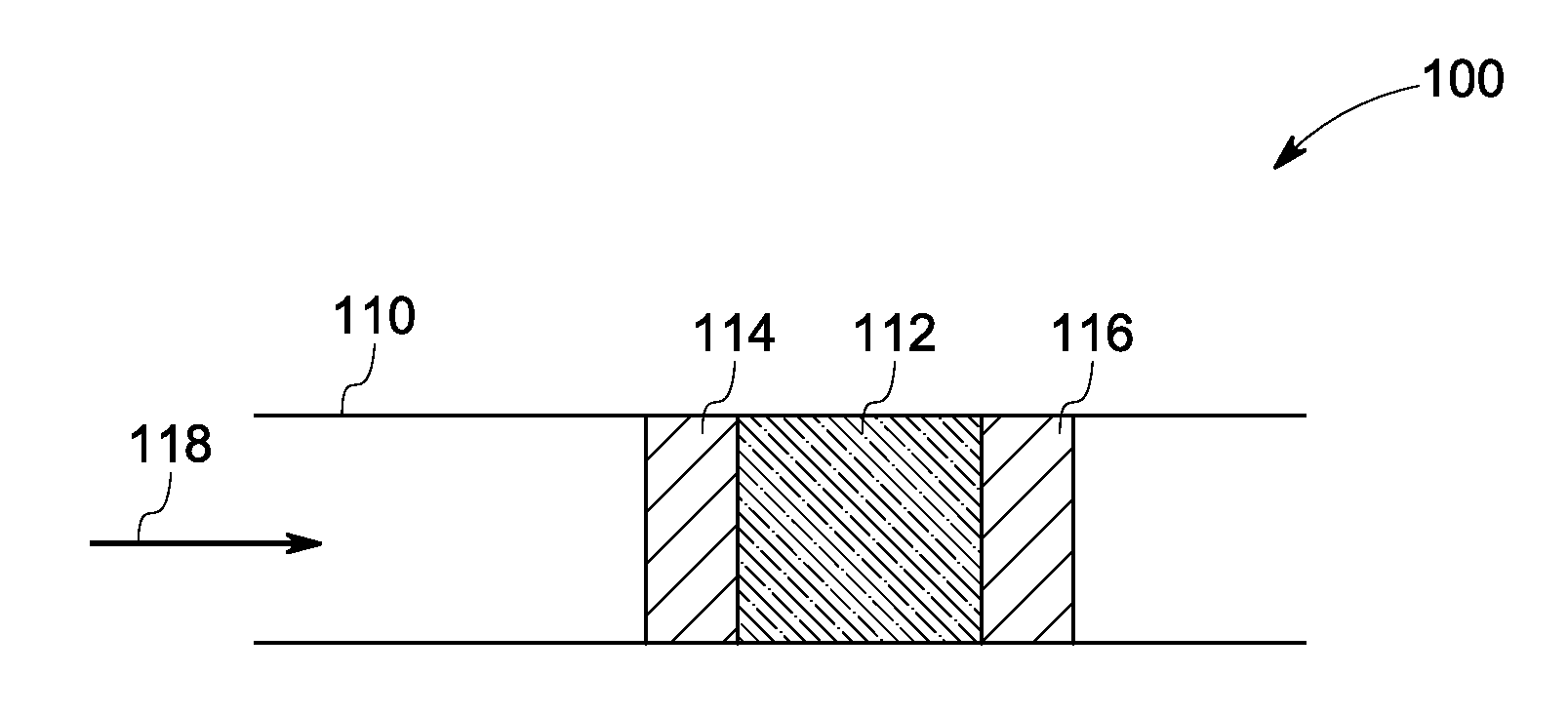
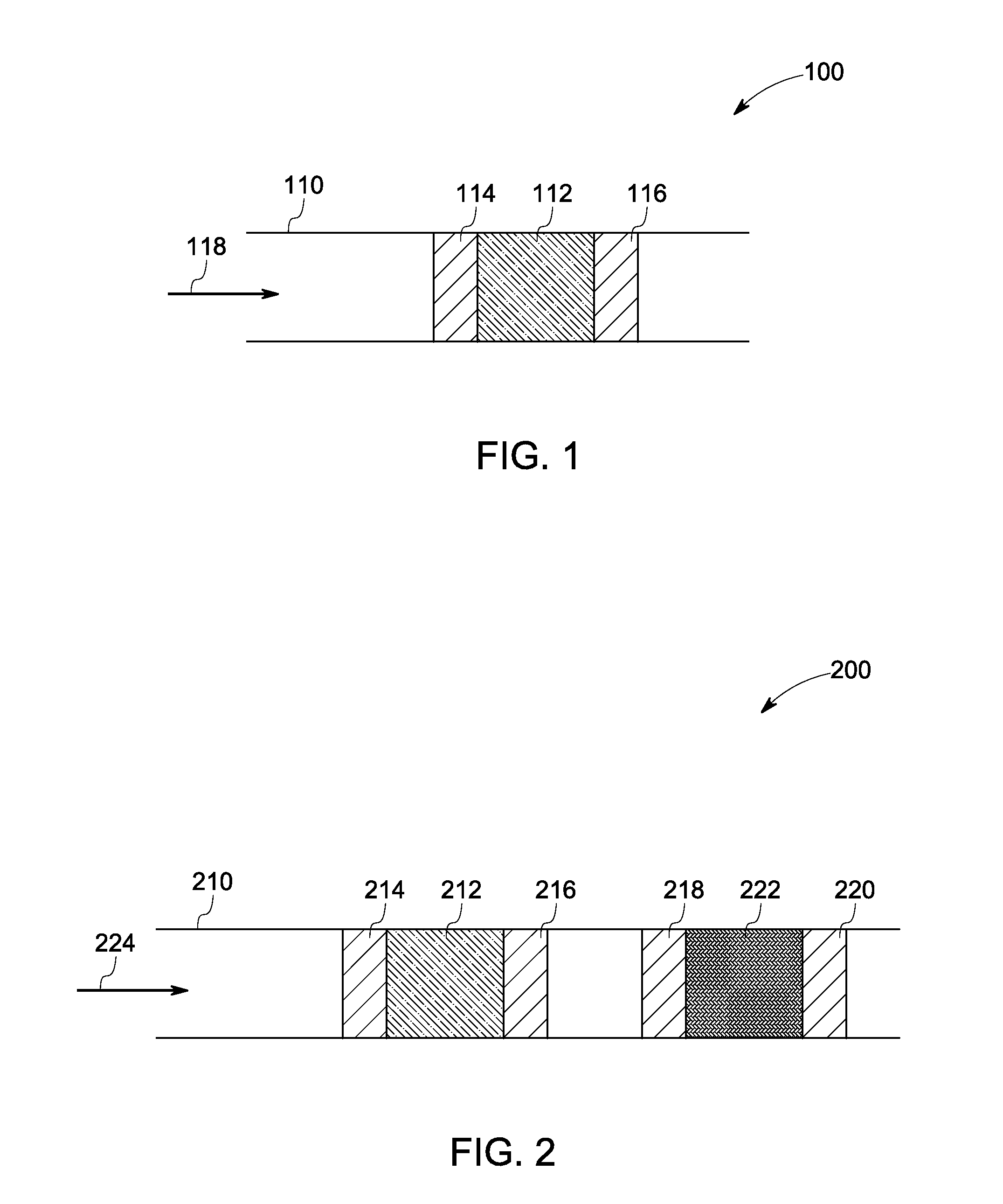
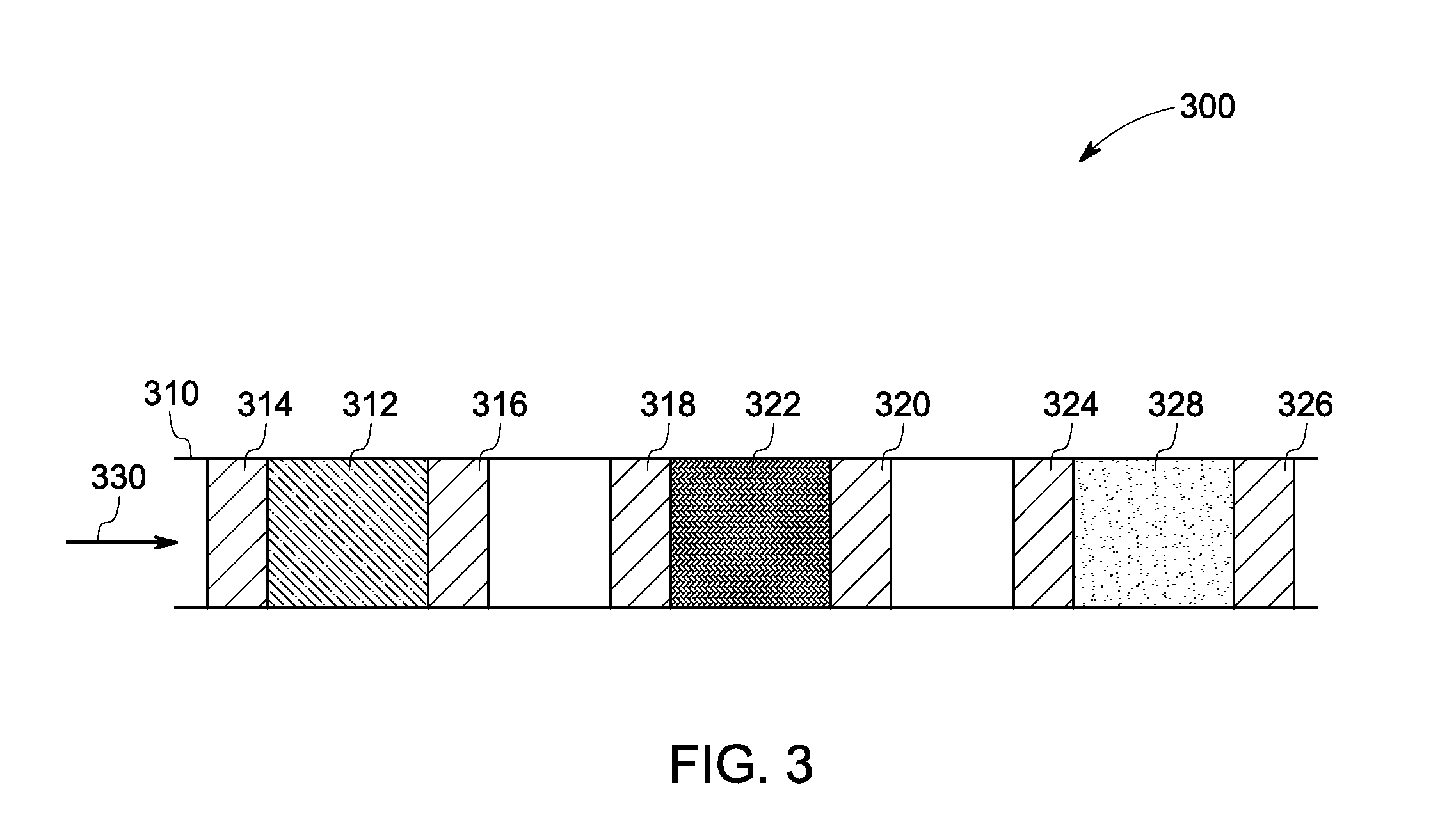
Catalyst and method of manufacture
a technology of catalysts and catalysts, applied in the field of catalysts, can solve the problems of ammonia slippage, ammonia solutions require an extra storage tank, and are subject to freezing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Silver Nanocrystals
[0102]To a 3-neck flask equipped with a stirrer was charged silver acetate (4 millimoles, SA), oleic acid (4 milliliters, OA, technical grade, 90 percent, Aldrich) and trioctylamine (15 milliliters, TOA) at a temperature of about 25 degrees C. (room temperature). The resultant mixture was heated under a vacuum of 1 millimeter of mercury to a temperature of about 60 degrees C. As the temperature increased the silver acetate started to dissolve in the trioctylamine. Bubbling was observed in the flask, due the boiling of water present as an impurity in the reaction material. The water was removed under vacuum in the form of water vapor. When the temperature rose to 60 degrees C., silver acetate was completely dissolved in the trioctylamine and the resultant solution turned to a brown-grey-black color, indicating the decomposition of silver acetate and the formation of silver nanocrystals. The temperature was then increased to 90 degrees C. and the mixt...
examples 2-3
Preparation of Silver Nanocrystals
[0107]Examples 2 and 3 were carried out in a manner similar to that described above in Example 1 except in that they were carried out in relatively larger scales. The amounts of silver acetate, oleic acid, and trioctylamine used and the resultant concentration of AgNC in hexane and PSD of AgNC are included in Table 3 below. Examples 2 and 3 show that a PSD of less that 10 percent can be achieved even in large scale batches.
TABLE 3Concentration ofSAOATOAAgNC in hexaneExample(millimoles)(milliliters)(milliliters)(grams per liter)PSD of AgNC1441532.4 to 53.9Less than 10 percent240408032.4 to 53.9Less than 10 percent310010020032.4 to 53.9Less than 10 percent
examples 4-7
Preparation of First Catalytic Composition
[0108]To a 3-neck flask equipped with a stirrer was charged aluminum (sec-butoxide)3 (50 grams) and IPA (200 milliliters). A predetermined amount of AgNC in hexane solution containing 43.1 grams (0.4 moles) AgNC in hexane, was then added to the flask to form a first solution. The predetermined amount of AgNC in hexane solution added and the resultant loading of AgNC obtained in the catalyst composition is included in Table 4 below. Following the addition to the flask, the mechanical stirrer was turned on using a speed of about 60 revolutions per minute in another separate flask, ethyl acetoacetate (2.65 grams), Triton X-114 (14 grams), and 65 milliliters isopropyl alcohol were added to form a second solution. The second solution was then poured into the first solution. The resultant mixture was stirred for about 30 minutes at a speed of about 180 revolutions per minute at a temperature of about 25 degrees C. During the 30-minute stir period,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com