Compositions and methods for treating precocious puberty
a technology of gonadotropin and agonist, applied in the field of precocious puberty, can solve the problems of gonad function at an inappropriately early age, affective individuals may experience psychological problems, and girls will often experience moodiness and become more irritable, so as to prevent the loss of gnrh agonis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
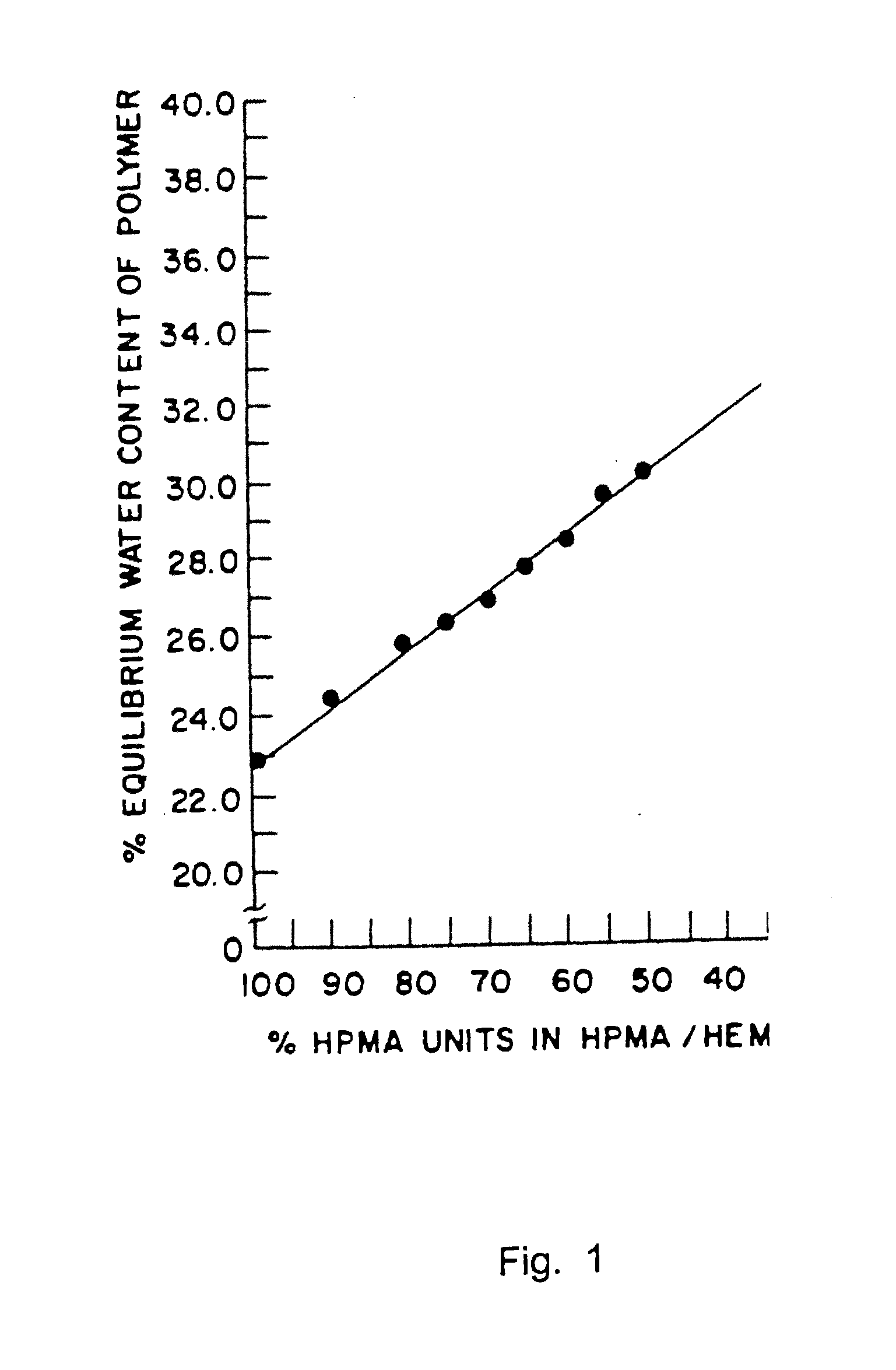
[0107]Hydrogel implants were prepared in accordance with the present invention. In particular, about 45% HEMA, 54.5% HPMA and about 0.5% TMPTMA were admixed with 0.3% benzoinmethylether (BME) and 0.1% percadox-16 .
example 2
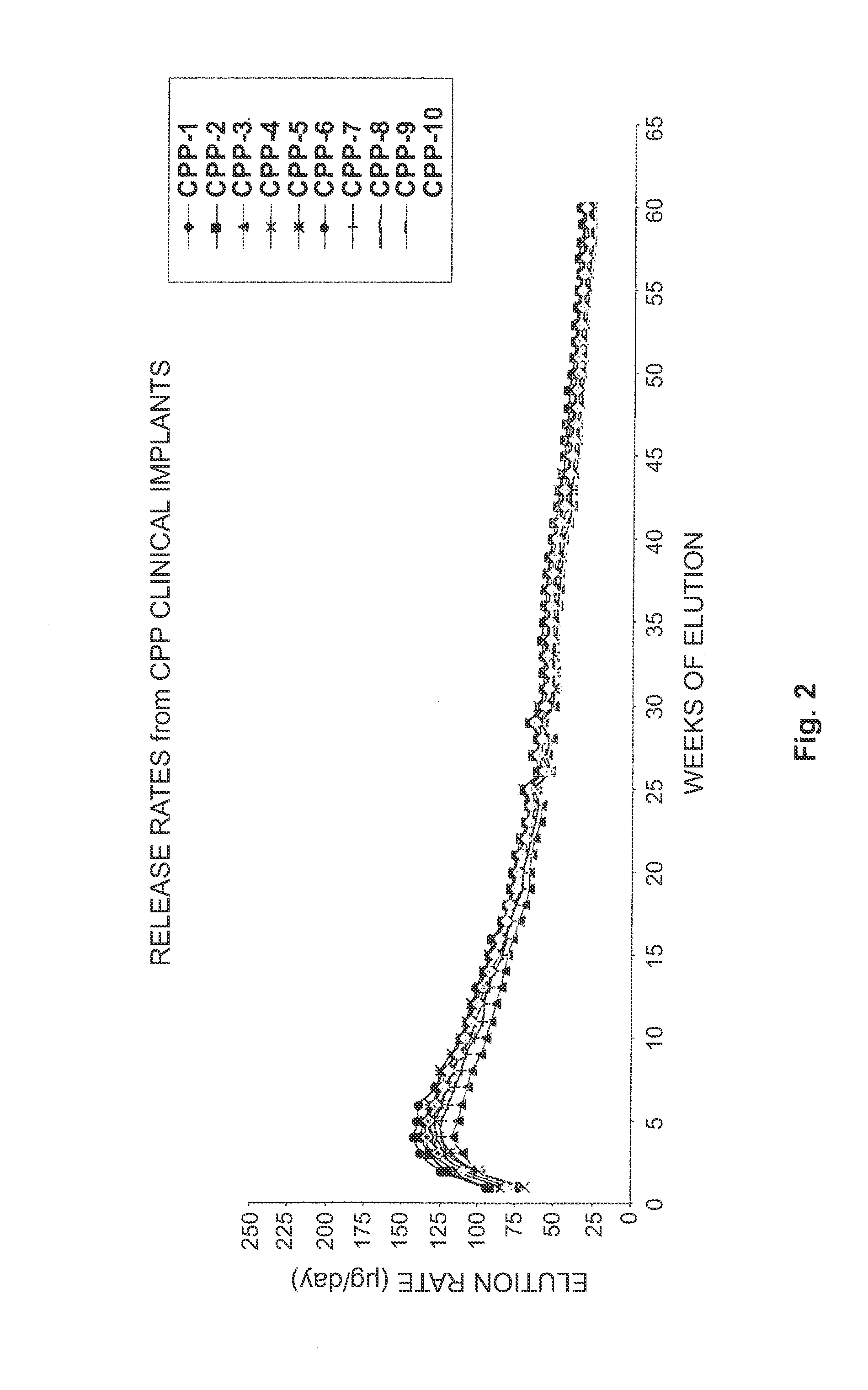
[0108]Ten histrelin implants were prepared in accordance with the present invention. In particular, the histrelin implants comprised about 45% HEMA and about 55% HPMA. The resulting hydrogel implants exhibited an equilibrium water content (EWC) of about 29%. The implants were prehydrated. The release rates were measured over sixty weeks. The release profile for the histrelin implants is provided in FIG. 2.
example 3
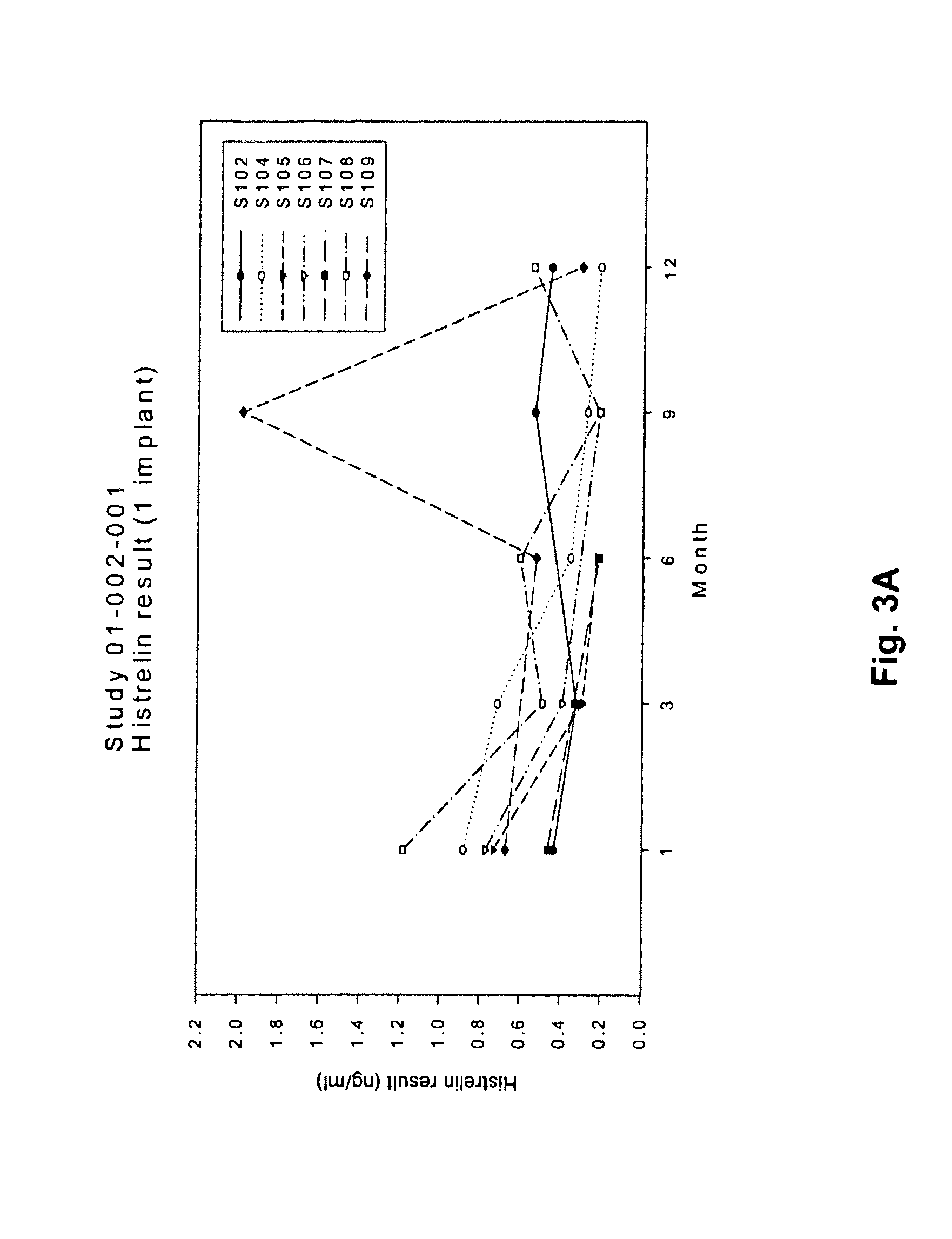
[0109]This was an open-label study in which children with CPP were assigned to receive one or two histrelin hydrogel implants, containing 50 mg of histrelin each, depending on their body weight and the ease of insertion of the implant, as determined on a case-by-case basis by the principal investigator and the surgeon performing the insertion. The histrelin implants were supplied in about 2.0 mL of 1.8% sodium chloride solution. Following the first 9 months of the study, the children were assigned to one of two tracks. Children in the first track had their implant(s) removed, and one new implant inserted. The implant remained in place in those in the second track. The children in both tracks continued in the study for an additional 9 months. In total, eleven girls ages 3 to 11 years old were enrolled in this study. Ten of the 11 patients completed at least 12 months of treatment.
[0110]Study visits occurred at baseline, 4 weeks (Month 1), 3 months, and then every 3 months for the rem...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com