Inhalation device for drugs in powder form
a technology of inhalation device and drug, which is applied in the direction of packaging, other medical devices, coatings, etc., can solve the problems of affecting the the breakdown of galenic powder into particles which can reach the lungs, and the disadvantageous effect of the pharmaceutically active medicament composition, etc., to achieve better dose dispensing, better reproducibility of the drug, and the effect of reducing the risk of infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
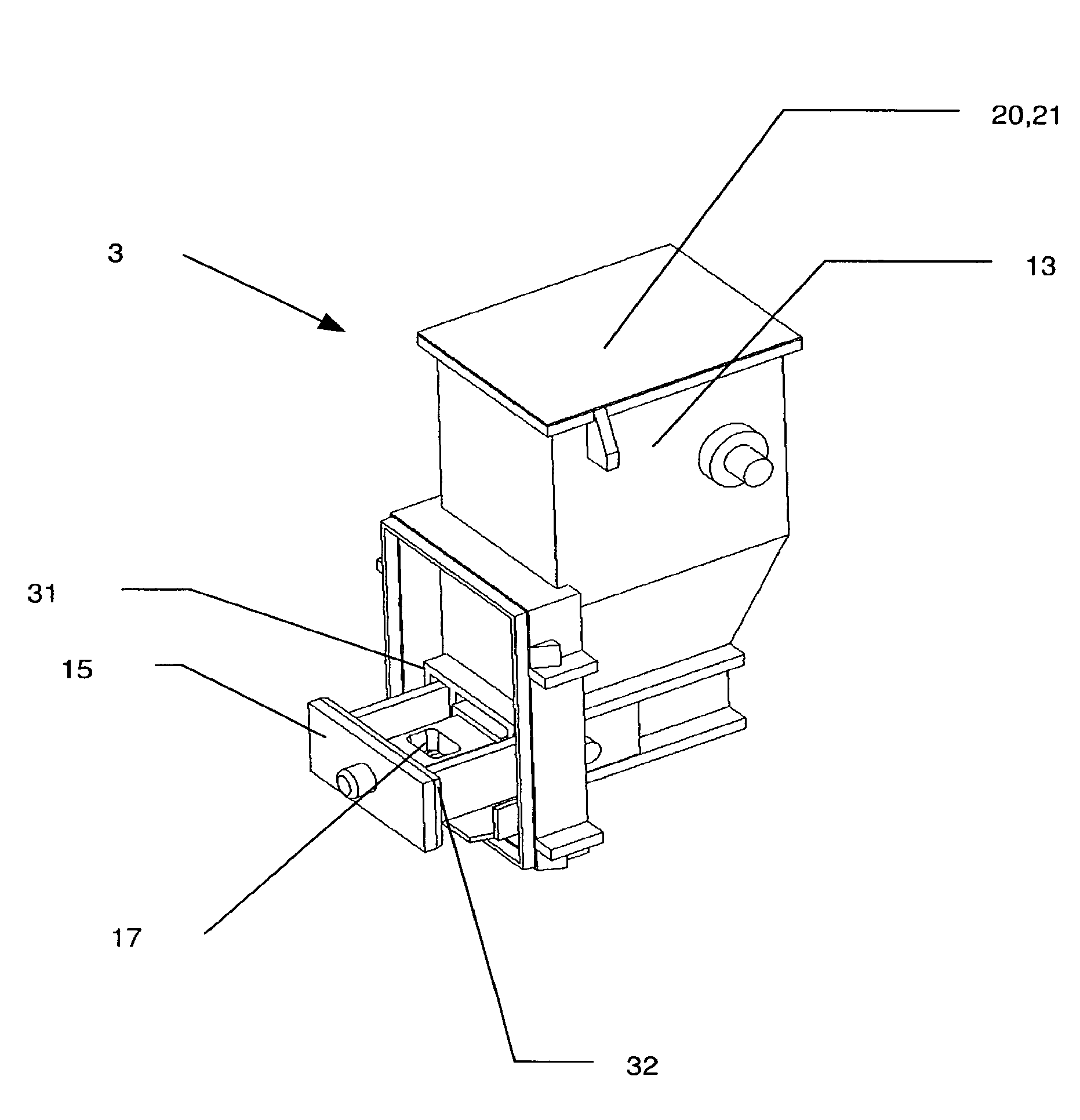
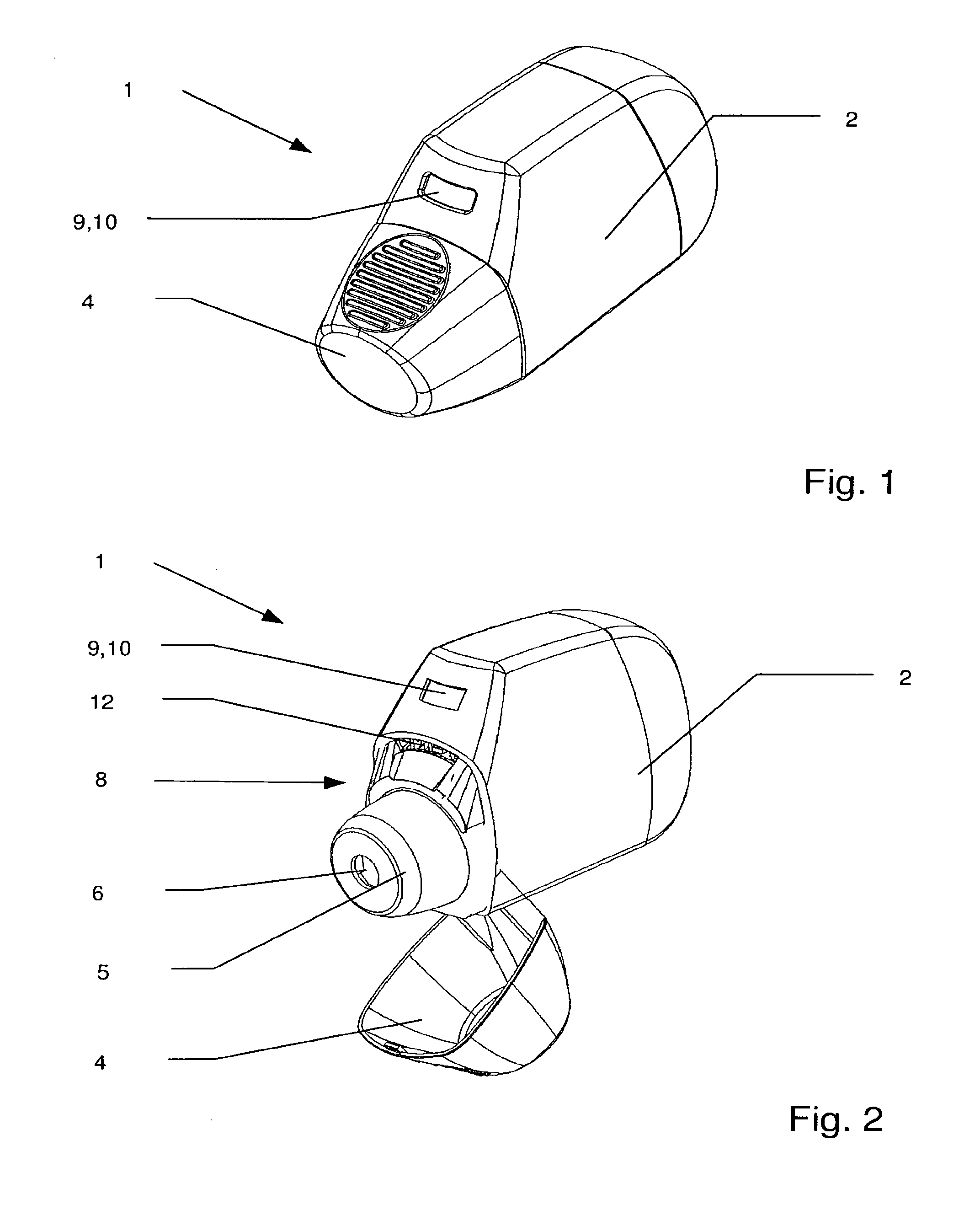
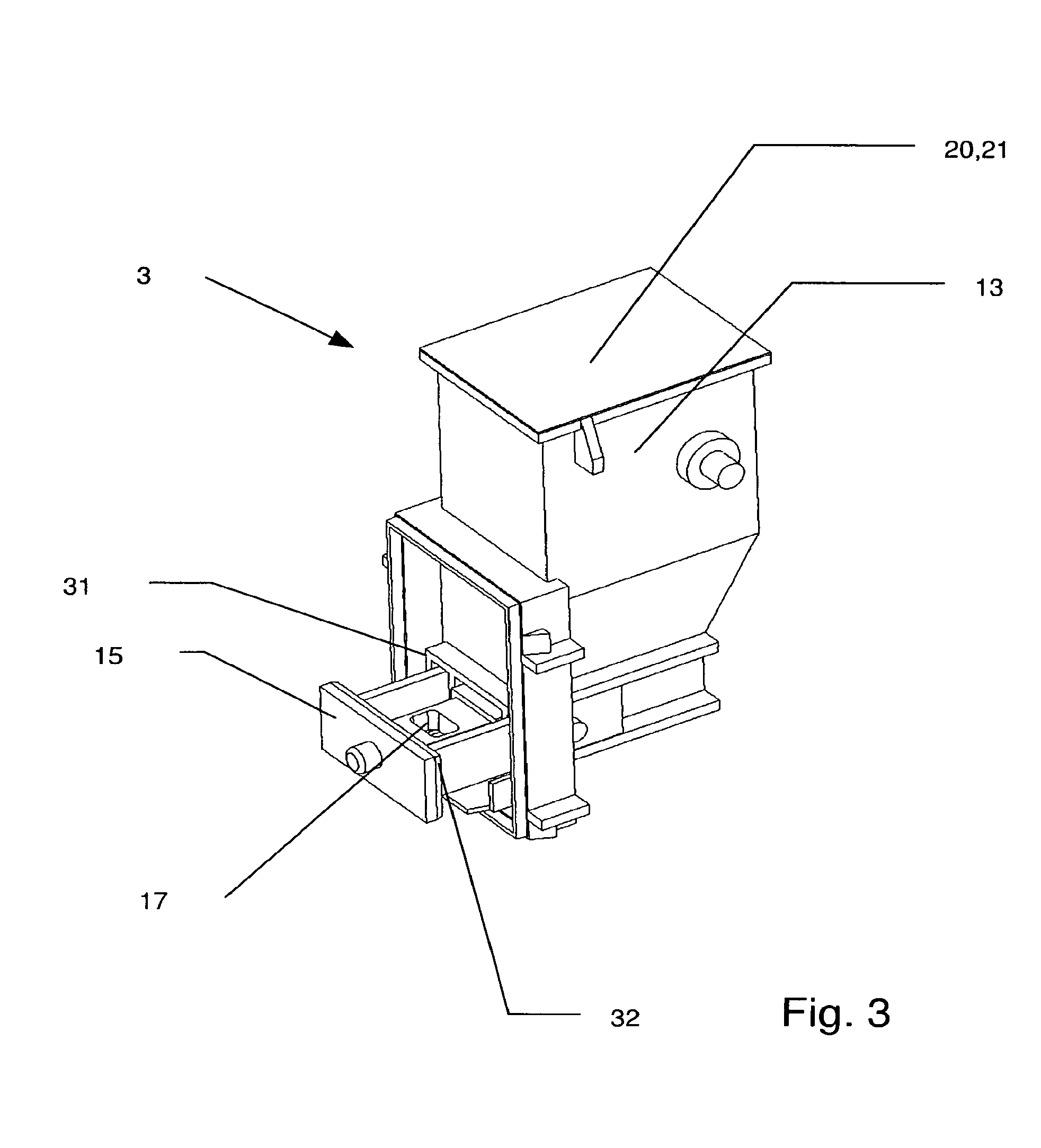
[0121]FIG. 1 shows a perspective view of an embodiment of an inhalation device according to the invention indicated generally by reference 1. Such an inhalation device 1 is also referred to as an inhaler. The inhalation device 1 according to the invention is provided for the delivery of a large number of individual doses of a drug in powder form. Special inhalation devices of that kind are therefore also referred to as powder inhalers and often abbreviated to MDPI (multi-dose powder inhaler). The inhaler 1 includes a housing 2. The housing 2 desirably comprises two halves with a separation line along the central axis along the inhaler 1. That means that the housing portions can be easily manufactured using plastic injection moulding and it has been found that, with such an arrangement, a powder inhaler 1 is simple to assemble. The housing 2 can also include a separate housing cover so that a separate drug powder cartridge 3 can be subsequently inserted into the assembled inhaler. Th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com