Multi-specific antigen-binding molecules and uses thereof
a technology of antigen binding molecules and specificity, applied in the direction of antibody medical ingredients, drug compositions, extracellular fluid disorders, etc., can solve the problems of insufficient activity of functionally substituting for f.viii, insufficient bypass formulations, etc., to achieve low f.xase inhibitory action, high activity, and high functional activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
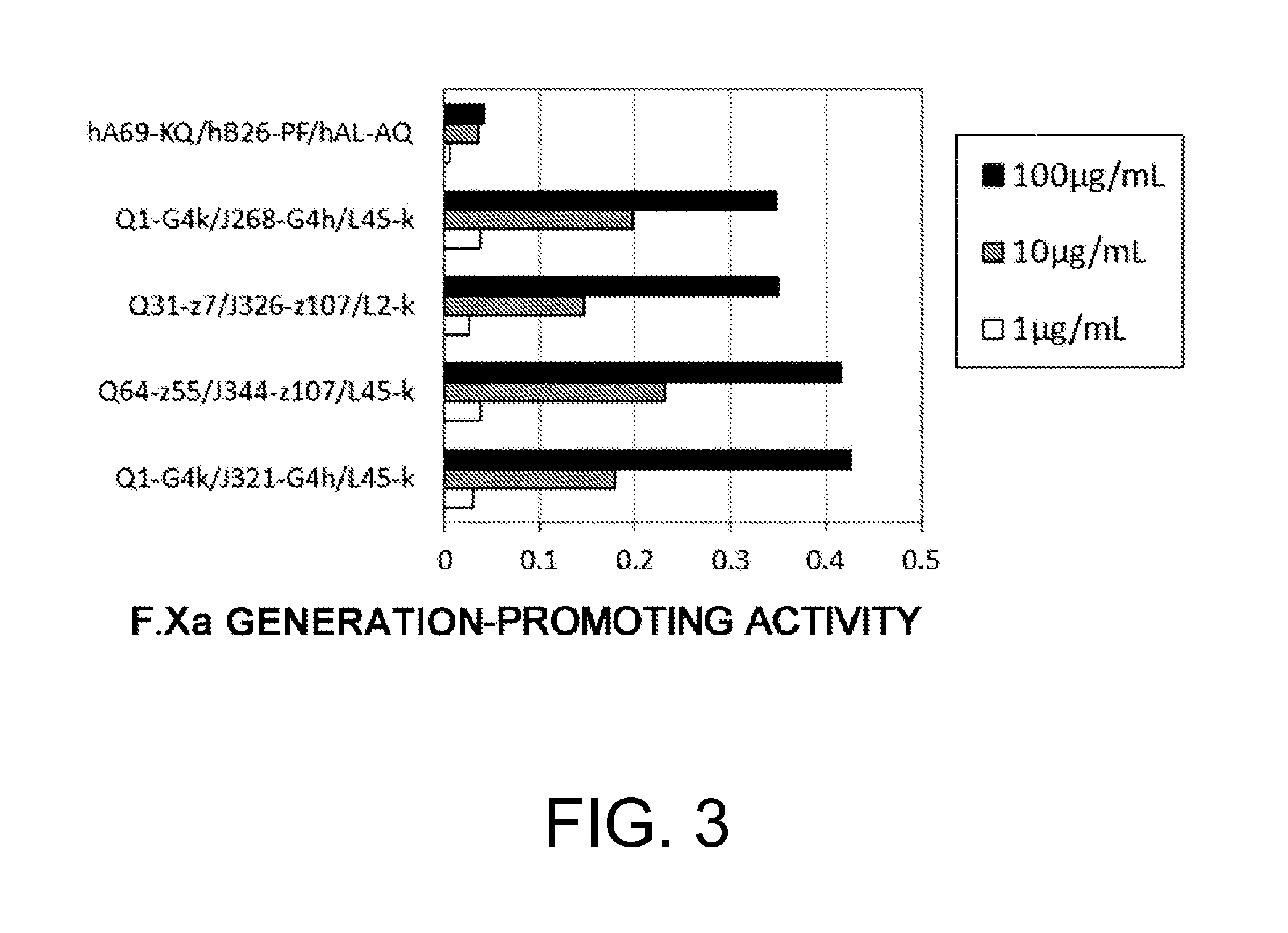
Production of Bispecific Antibodies Having F.Xa Generation-Promoting Activity
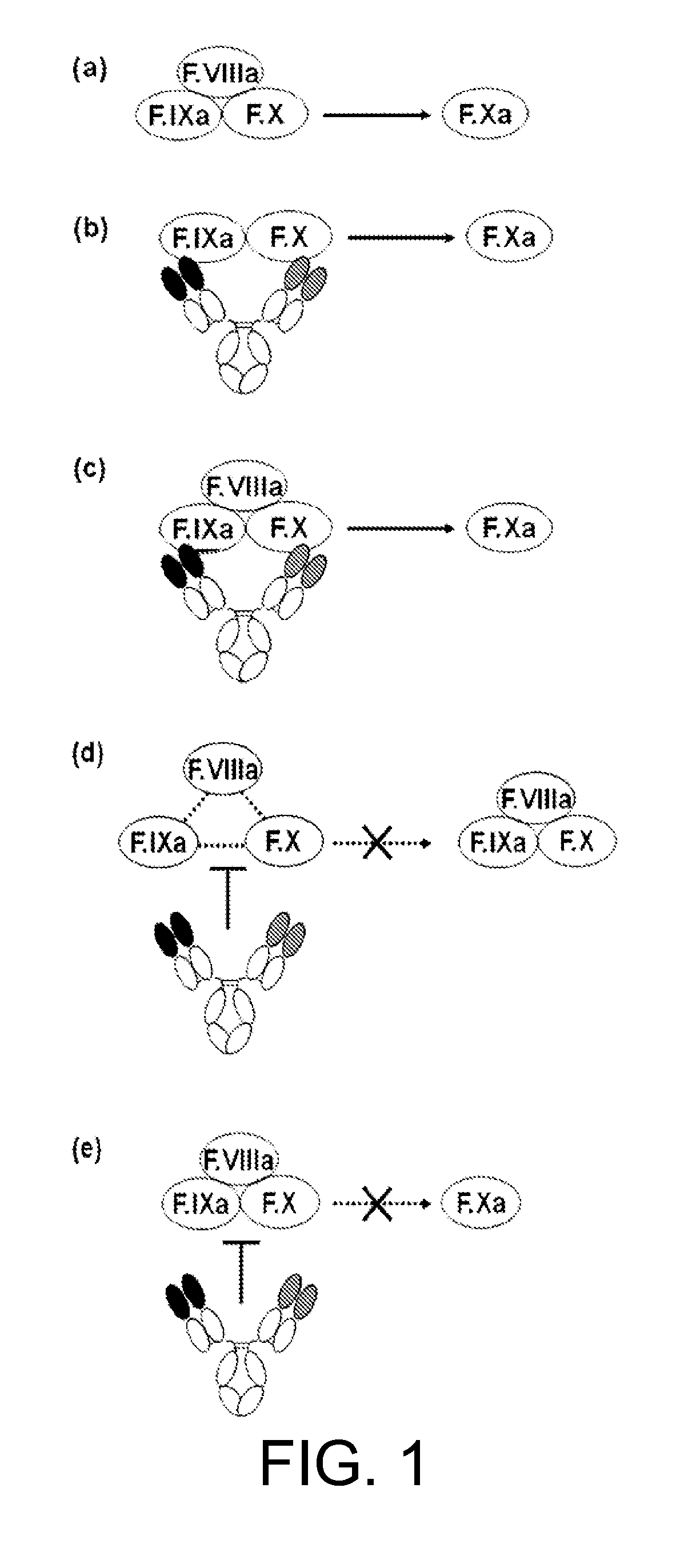
[0507]In WO 2006 / 109592, hA69-KQ / hB26-PF / hAL-AQ was obtained as a bispecific antibody having an activity of functionally substituting for F.VIII. However, there was the possibility that this antibody has an inhibiting action on the reaction in which F.IXa activates F.X using F.VIIIa as a cofactor.
[0508]As shown in FIG. 1, antibodies that bind to F.IX / F.IXa or F.X may inhibit the formation of the F.IXa-F.VIIIa complex (Factor Xase (F.Xase)), or inhibit F.Xase activity (activation of F.X). Hereafter, inhibition of F.Xase formation and / or action of inhibiting F.Xase activity will be mentioned as F.Xase inhibitory action. F.Xase inhibitory action is the inhibition of a coagulation reaction in which F.VIIIa serves as the cofactor, which may suppress the remaining F.VIII function in a patient or the function of the administered F.VIII formulation. Therefore, it is desirable that F.Xa generation-promoting activity...
example 2
Production of Modified Antibodies
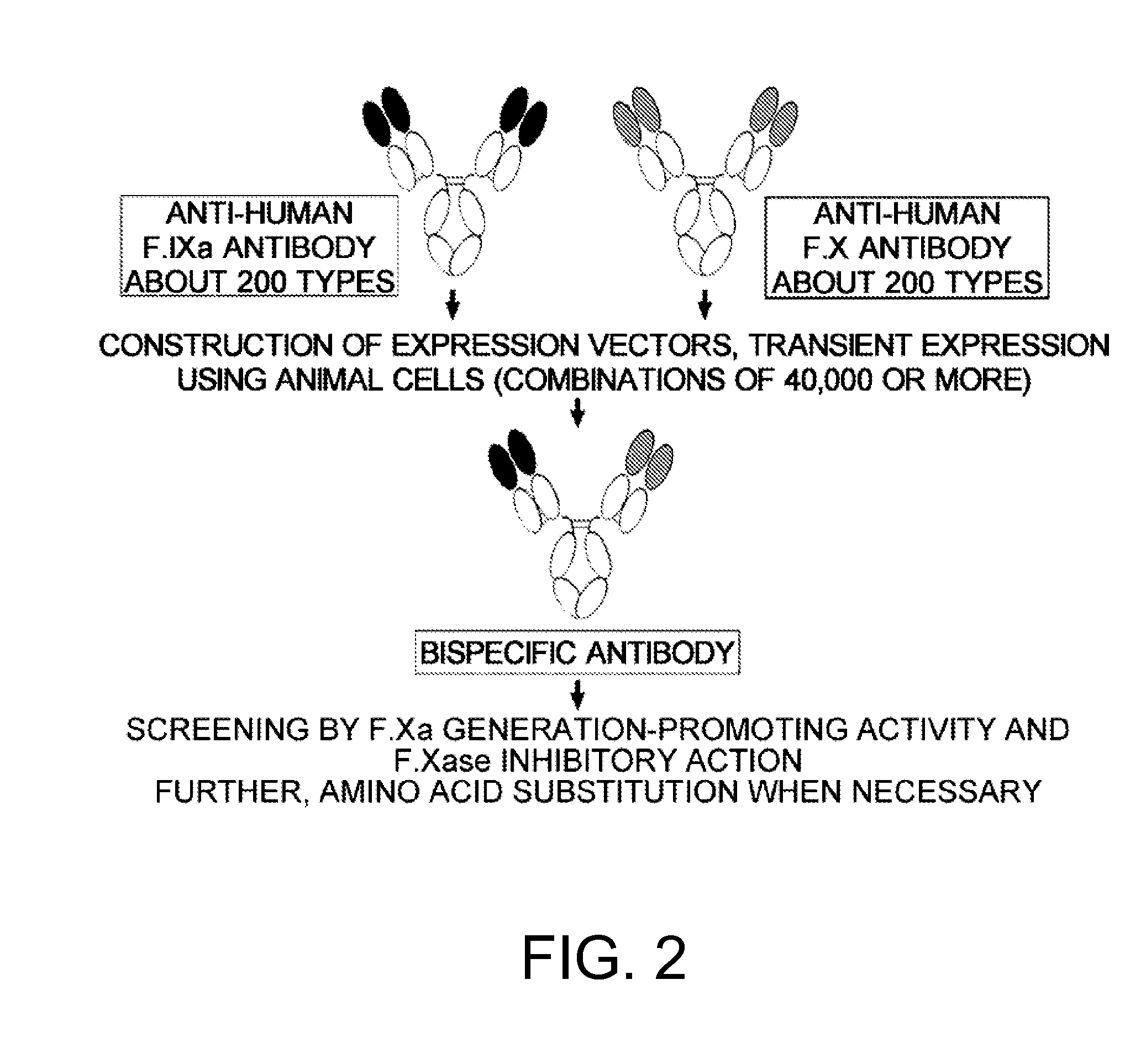
[0525]The present inventors introduced various combinations of amino acid mutations that affect the F.Xa generation-promoting activities and F.Xase inhibitory actions found in Example 1 to each of the chains of the prototype antibodies by a method known to those skilled in the art such as PCR for introducing mutations and evaluated the combinations of modified chains on a large scale to screen for amino acid substitutions that will further increase the F.Xa generation-promoting activities and reduce the F.Xase inhibitory actions of the four prototype antibodies.
[0526]Each of the modified bispecific antibodies with amino acid substitutions were expressed transiently and purified by methods similar to those for the prototype antibodies. The F.Xa generation-promoting activities of the antibodies were measured using the following method. All reactions were performed at room temperature.
[0527]Five μL of antibody solution diluted with Tris-buffered saline ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com