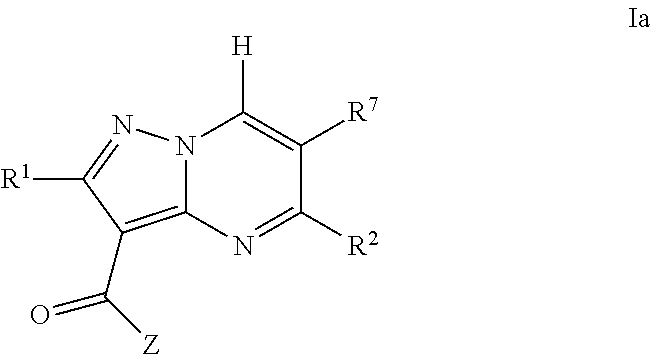
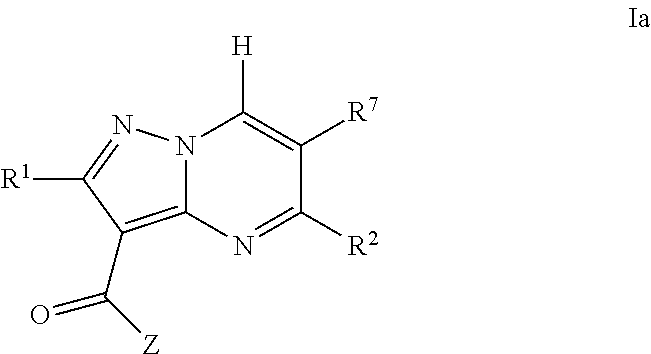
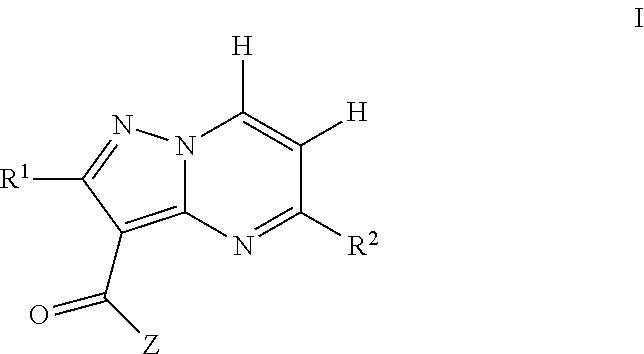
Pyrazolopyrimidine jak inhibitor compounds and methods
a technology of pyrazolopyrimidine and inhibitor compounds, applied in the field of p, can solve the problems of life-threatening thrombotic events, no known cure for ph-mpd diseases, etc., and achieve the effect of lessening the severity of a diseas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example a
JAK2 Inhibition Assay Protocol
[0310]The activity of the isolated JAK2 kinase domain was measured by monitoring phosphorylation of a peptide derived from JAK3 (Val-Ala-Leu-Val-Asp-Gly-Tyr-Phe-Arg-Leu-Thr-Thr) fluorescently labeled on the N-terminus with 5-carboxyfluorescein using the Caliper LabChip technology (Caliper Life Sciences, Hopkinton, Mass.). To determine the inhibition constants (Ki), compounds were diluted serially in DMSO and added to 50 μL kinase reactions containing 0.2 nM purified JAK2 enzyme, 100 mM Hepes pH7.2, 0.015% Brij-35, 1.5 μM peptide substrate, 25 μM ATP, 10 mM MgCl2, 4 mM DTT at a final DMSO concentration of 2%. Reactions were incubated at 22° C. in 384-well polypropylene microtiter plates for 30 minutes and then stopped by addition of 25 μL of an EDTA containing solution (100 mM Hepes pH 7.2, 0.015% Brij-35, 150 mM EDTA), resulting in a final EDTA concentration of 50 mM. After termination of the kinase reaction, the proportion of phosphorylated product was...
example b
JAK1 and TYK2 Inhibition Assay Protocol
[0311]The activity of the isolated JAK1 or TYK2 kinase domain was measured by monitoring phosphorylation of a peptide derived from JAK3 (Val-Ala-Leu-Val-Asp-Gly-Tyr-Phe-Arg-Leu-Thr-Thr) fluorescently labeled on the N-terminus with 5-carboxyfluorescein using the Caliper LabChip technology (Caliper Life Sciences, Hopkinton, Mass.). To determine inhibition constants (Ki), compounds were diluted serially in DMSO and added to 50 uL kinase reactions containing 1.5 nM JAK1, 0.2 nM purified JAK2 or 1 nM purified TYK2 enzyme, 100 mM Hepes pH7.2, 0.015% Brij-35, 1.5 uM peptide substrate, 25 uM ATP, 10 mM MgCl2, 4 mM DTT at a final DMSO concentration of 2%. Reactions were incubated at 22° C. in 384-well polypropylene microtiter plates for 30 minutes and then stopped by addition of 25 uL of an EDTA containing solution (100 mM Hepes pH 7.2, 0.015% Brij-35, 150 mM EDTA), resulting in a final EDTA concentration of 50 mM. After termination of the kinase reacti...
example c
JAK3 Inhibition Assay Protocol
[0312]The activity of the isolated JAK3 kinase domain was measured by monitoring phosphorylation of a peptide derived from JAK3 (Leu-Pro-Leu-Asp-Lys-Asp-Tyr-Tyr-Val-Val-Arg) fluorescently labeled on the N-terminus with 5-carboxyfluorescein using the Caliper LabChip technology (Caliper Life Sciences, Hopkinton, Mass.). To determine inhibition constants (Ki), compounds were diluted serially in DMSO and added to 50 uL kinase reactions containing 5 nM purified JAK3 enzyme, 100 mM Hepes pH7.2, 0.015% Brij-35, 1.5 uM peptide substrate, 5 uM ATP, 10 mM MgCl2, 4 mM DTT at a final DMSO concentration of 2%. Reactions were incubated at 22° C. in 384-well polypropylene microtiter plates for 30 minutes and then stopped by addition of 25 uL of an EDTA containing solution (100 mM Hepes pH 7.2, 0.015% Brij-35, 150 mM EDTA), resulting in a final EDTA concentration of 50 mM. After termination of the kinase reaction, the proportion of phosphorylated product was determined...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com