Composition for regeneration of cartilage
a cartilage and collagen technology, applied in the direction of drugs, peptide/protein ingredients, prosthesis, etc., can solve the problems of lack of vascular and neural networks, lack of cartilage defect lesion repair ability, etc., to reduce the burden on patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
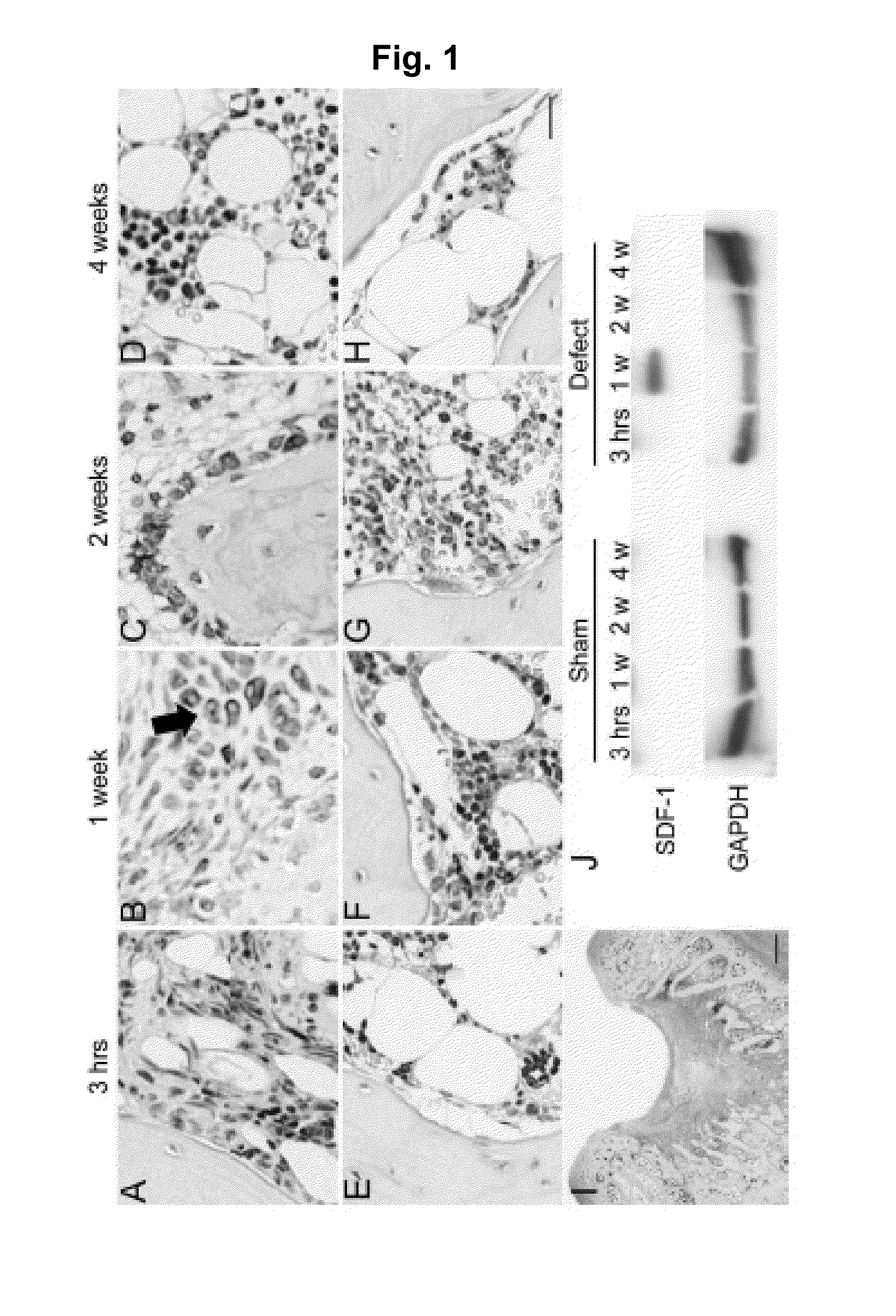
Expression of SDF-1 Protein in Osteochondral Defect Models
[0118]An osteochondral defect model of a rabbit knee joint was used to examine change in the expression of SDF-1 protein at a injury site with time by immunohistostaining
[0119]15-week-old female Japanese white rabbits (weight 2.6 to 2.9 kg) were used to prepare osteochondral defect models. Anesthesia was performed by intravenous administration of 0.05 mg / kg pentobarbital and inhalation anesthesia of isoflurane. An antibiotic (penicillin G, Meiji Co., Ltd.) was intramuscularly administered. After incision in the skin of about 2 cm, patella was turned over by medial parapatellar approach to expand the patellar surface of the femur. A power drill (Rexon, Kawasaki) was used to create full-thickness osteochondral defects (diameter of 4.5 mm and depth of 3 mm) that reach the subchondral bones in the patellar surfaces of femurs of both knees. A sham surgery group (control) received the same surgery except the creation of the full-th...
example 2
Application of Alginic Acid Gel to Rabbit Osteochondral Defect Models
[0124](2-1) Alginic Acid
[0125]A low endotoxin sodium alginate (Sea Matrix (sterilized), with a molecular weight of about 1,700,000 Da, distributed from Mochida International Ltd.) was used. 12.5 ml of deionized water sterilized by a filter procedure was added to 0.25 g of sodium alginate (lyophilized product) to obtain a 2% sodium alginate solution that is to be used in the experiment. The endotoxin level of the low endotoxin sodium alginate was 5.76 EU (endotoxin unit) / g. The endotoxin level of food grade (commercial grade) sodium alginate (Wako Pure Chemical Industries, Ltd., sodium alginate 500, 199-09961) is 75,950 EU / g, and thus sodium alginate with an extremely low endotoxin level is used in this experiment.
[0126](2-2) Preparation of Models
[0127]Rabbit osteochondral defect models were prepared by the procedure described in Example 1. The prepared full-thickness osteochondral defects were washed with physiolog...
example 3
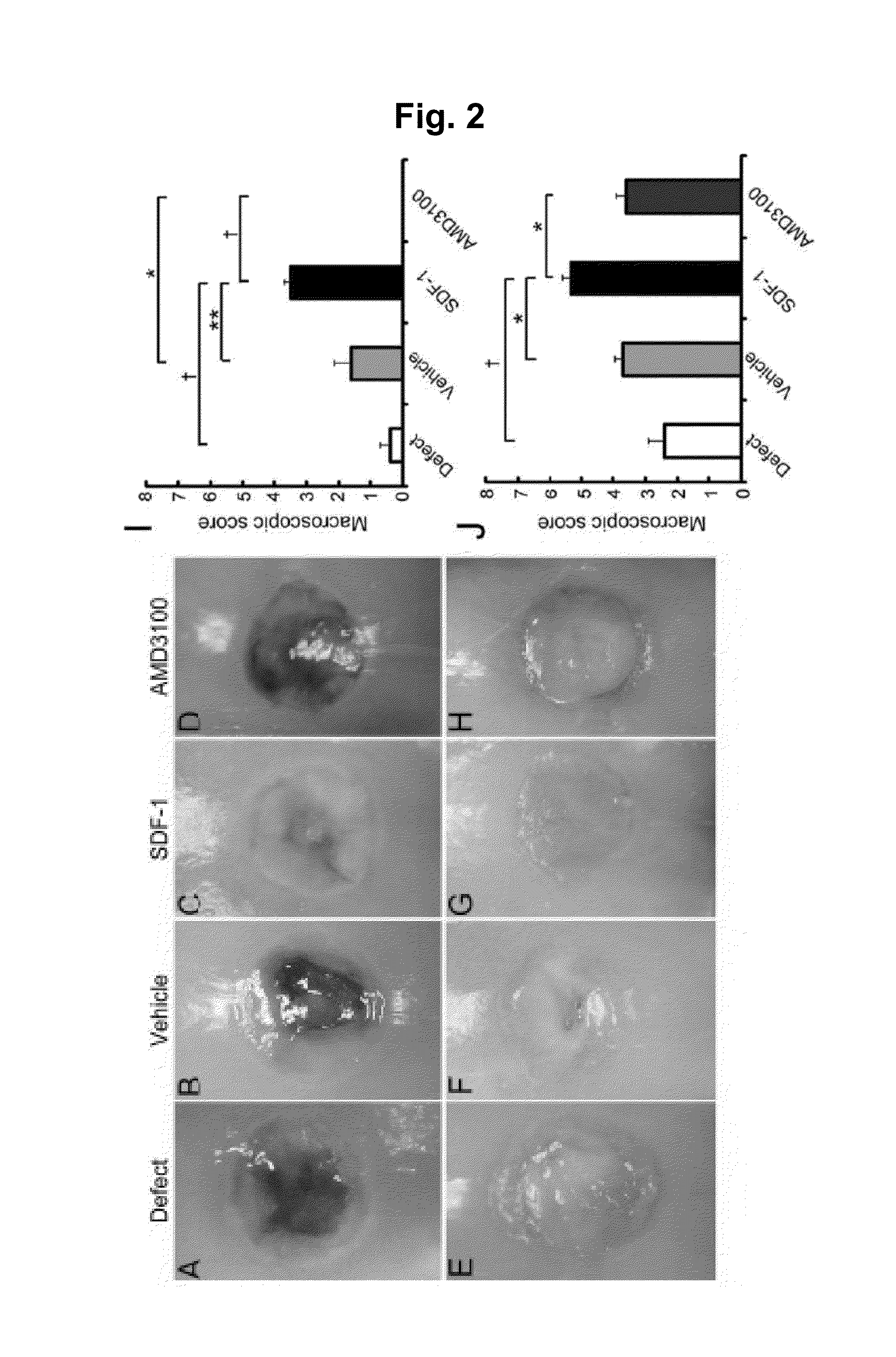
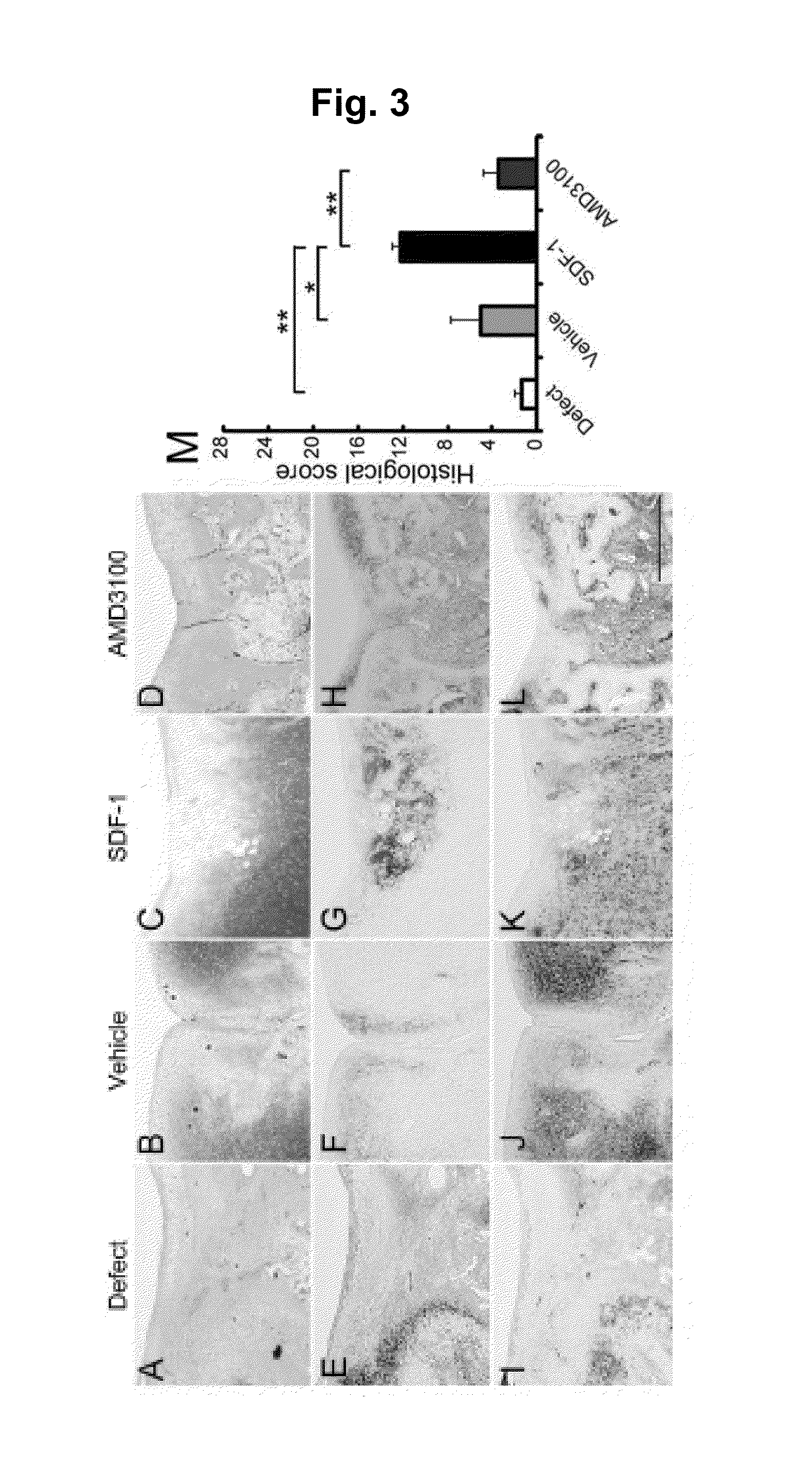
Assessment of In Vivo Cell Homing
[0156]After applying the alginic acid+SDF-1 to the osteochondral defect lesion, quantitative assessment was conducted to see how the in vivo cell homing and migration to the injury lesion take place. An alginic acid group, an alginic acid+SDF-1 administration group and an alginic acid+AMD3100 administration group were prepared in the same method as the rabbit osteochondral defect models described in Example 2. Seven days after the surgery, the implanted alginic acid gels were collected and the numbers of cells in the gels were counted. The collected gel was immobilized with 4% phosphate buffered paraformaldehyde for 24 hours and embedded in paraffin to prepare a sample using a section from the center of the gel with a thickness of 5 μm. The samples were stained with H-E (hematoxylin and eosin).
[0157]A week after the surgery, more cells were found in the gel of alginic acid+SDF-1 administration group compared to other groups (FIG. 7C). From the quanti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com