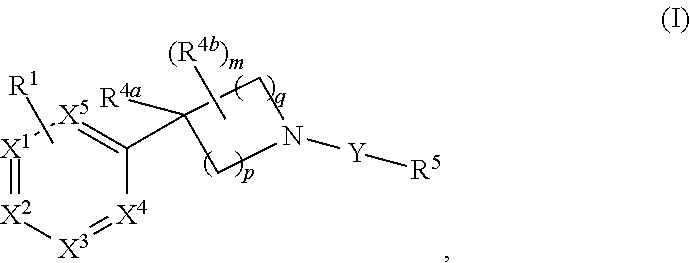
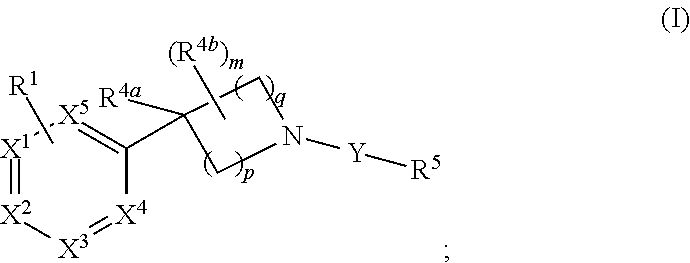
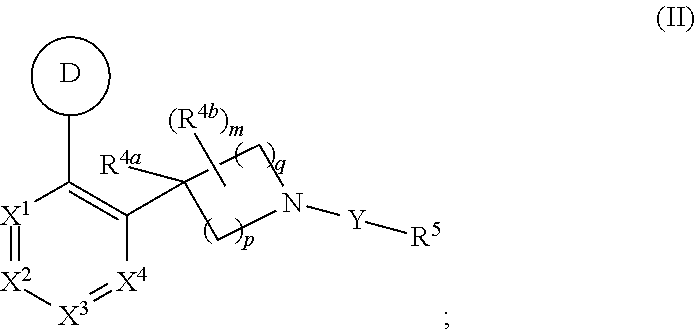
Unsaturated nitrogen heterocyclic compounds useful as pde10 inhibitors
a technology of unsaturated nitrogen and heterocyclic compounds, applied in the field of unsaturated nitrogen heterocyclic compounds, can solve the problem that a large amount of information cannot be obtained by other means
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1.1
(1H-BENZOIMIDAZOL-2-YL)-[3-(3-PHENYL-PYRIDIN-2-YL)-AZETIDIN-1-YL]-METHANONE
[0561]To a mixture of 1H-benzoimidazole-2-carboxylic acid (124 mg, 0.76 mmol) in DMF (5 mL) was added TEA (152 mg, 1.5 mmol) and HATU (347 mg, 0.92 mmol). The reaction mixture was stirred for 5 min and 2-azetidin-3-yl-3-phenyl-pyridine hydrochloride (100 mg, 0.76 mmol) was added. The reaction mixture was stirred at RT overnight. The mixture was diluted with water (10 mL), and extracted with EtOAc (2×20 mL). The combined organic extracts were washed with water (5 mL) and brine (5 mL), dried over Na2SO4, and filtered. The filtrate was evaporated in vacuo and the residue was purified by column chromatography to give (1H-benzoimidazol-2-yl)-[3-(3-phenyl-pyridin-2-yl)-azetidin-1-yl]-methanone (100 mg, 0.28 mmol, 59% yield) as a light yellow solid.
[0562]The following Table 13A lists compounds of Examples 1.1 to 1.17, which were made analogous to Scheme 1 by using the appropriate materials and reaction conditions, w...
example 2.1
(1-METHYL-1H-BENZOIMIDAZOL-2-YL)-[3-(3-PHENYL-PYRAZIN-2-YL)-AZETIDIN-1-YL]-METHANONE
[0563]To a stirred solution of [3-(3-chloro-pyrazin-2-yl)-azetidin-1-yl]-(1-methyl-1H-benzoimidazol-2-yl)-methanone (100 mg, 0.30 mmol) in dioxane (10 mL) was added phenylboronic acid (87 mg, 0.71 mmol), Na2CO3 (152 mg, 1.4 mmol) and H2O (2 mL). The reaction mixture was degassed with N2 and then PdCl2(dppf) (35 mg, 0.05 mmol) was added. The reaction mixture was stirred at 80° C. for 12 h. The reaction mixture was left to reach RT and filtered through a pad of CELITE® and the filter cake was washed with CH2Cl2 (20 mL×3). The combined filtrates were evaporated in vacuo and the residue was purified by column chromatography to give the desired compound (60 mg, 0.17 mmol, yield 70%)
[0564]The following Table 14A lists compounds of Examples 2.1 to 2.30, which were made analogous to Scheme 2 by using the appropriate materials and reaction conditions, which are listed in Table 14B. The NMR data of the Example...
example 3.1
(1-METHYL-1H-BENZOIMIDAZOL-2-YL)-[3-(3-PIPERIDIN-1-YL-PYRAZIN-2-YL)-AZETIDIN-1-YL]-METHANONE
[0565]To a mixture of [3-(3-chloro-pyrazin-2-yl)-azetidin-1-yl]-(1-methyl-1H-benzoimidazol-2-yl)-methanone (0.10 g, 0.30 mmol), piperidine (0.052 g, 0.60 mmol) and triethylamine (0.091 g, 0.90 mmol) was added DMSO (4 mL). The solution was heated to 120° C. for 5 h. Then the mixture was diluted with water (10 mL) and extracted with EtOAc (2×20 mL). The combined organic extracts were washed with water (10 mL), brine (10 mL), dried over Na2SO4 and filtered. The filtrate was evaporated in vacuo and the residue was purified by flash column chromatography on silica gel (20% to 50% EtOAc in petroleum ether) to give (1-methyl-1H-benzoimidazol-2-yl)-[3-(3-piperidin-1-yl-pyrazin-2-yl)-azetidin-1-yl]-methanone (0.072 g, 0.19 mmol, 63% yield) as white solid.
[0566]The following Table 15A lists compounds of Examples 3.1 to 3.54, which were made analogous to Scheme 3 by using the appropriate materials and r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ra | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com