Fixation of orthopaedic devices
a technology for orthopaedic devices and fixation, which is applied in the direction of prosthesis, ligaments, impression caps, etc., can solve the problems of removing the implant, loosing the implant, and ultimately detaching the implant, etc., and may be required for revision surgery, removal of the implant, and removal of the implan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
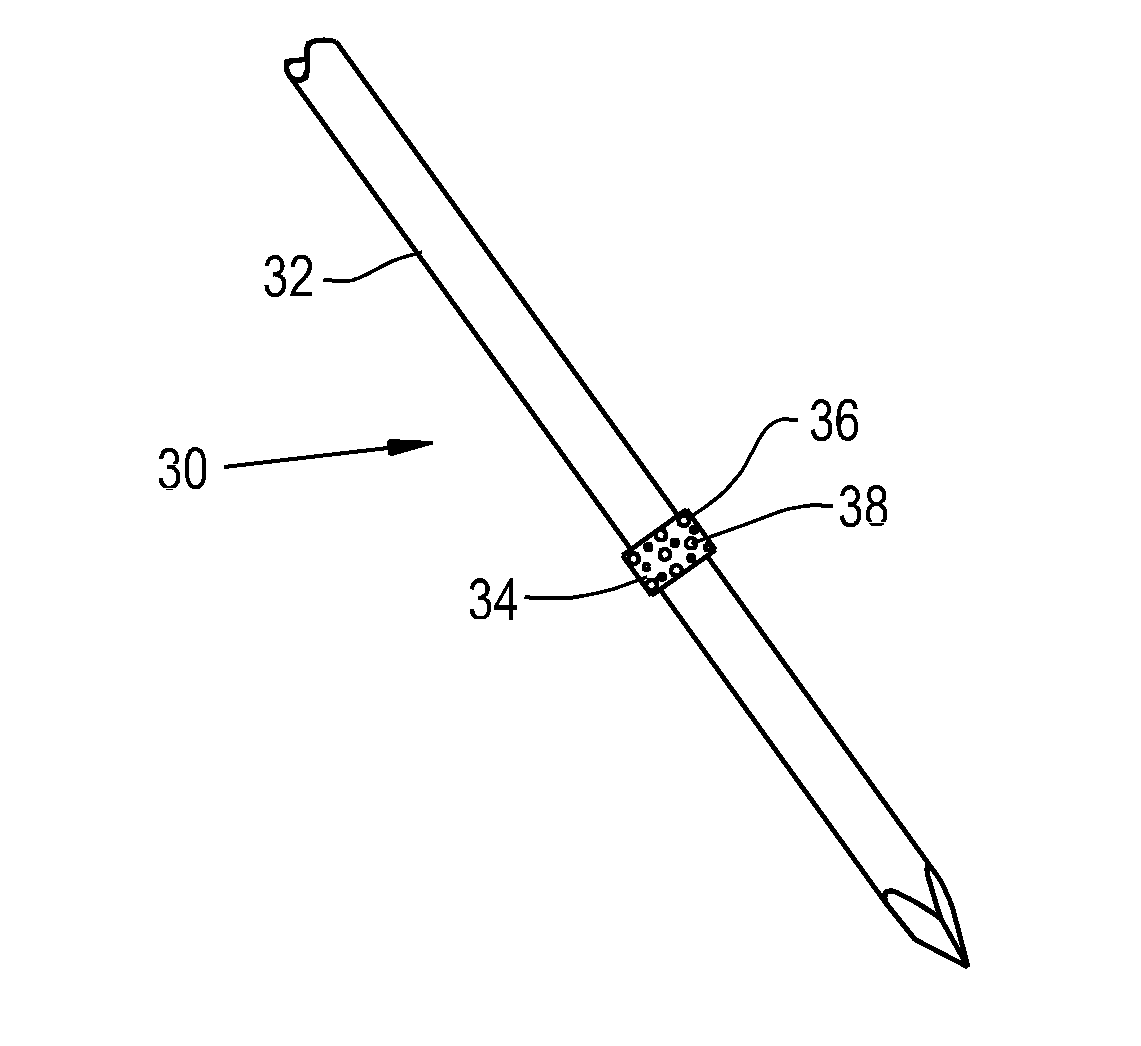
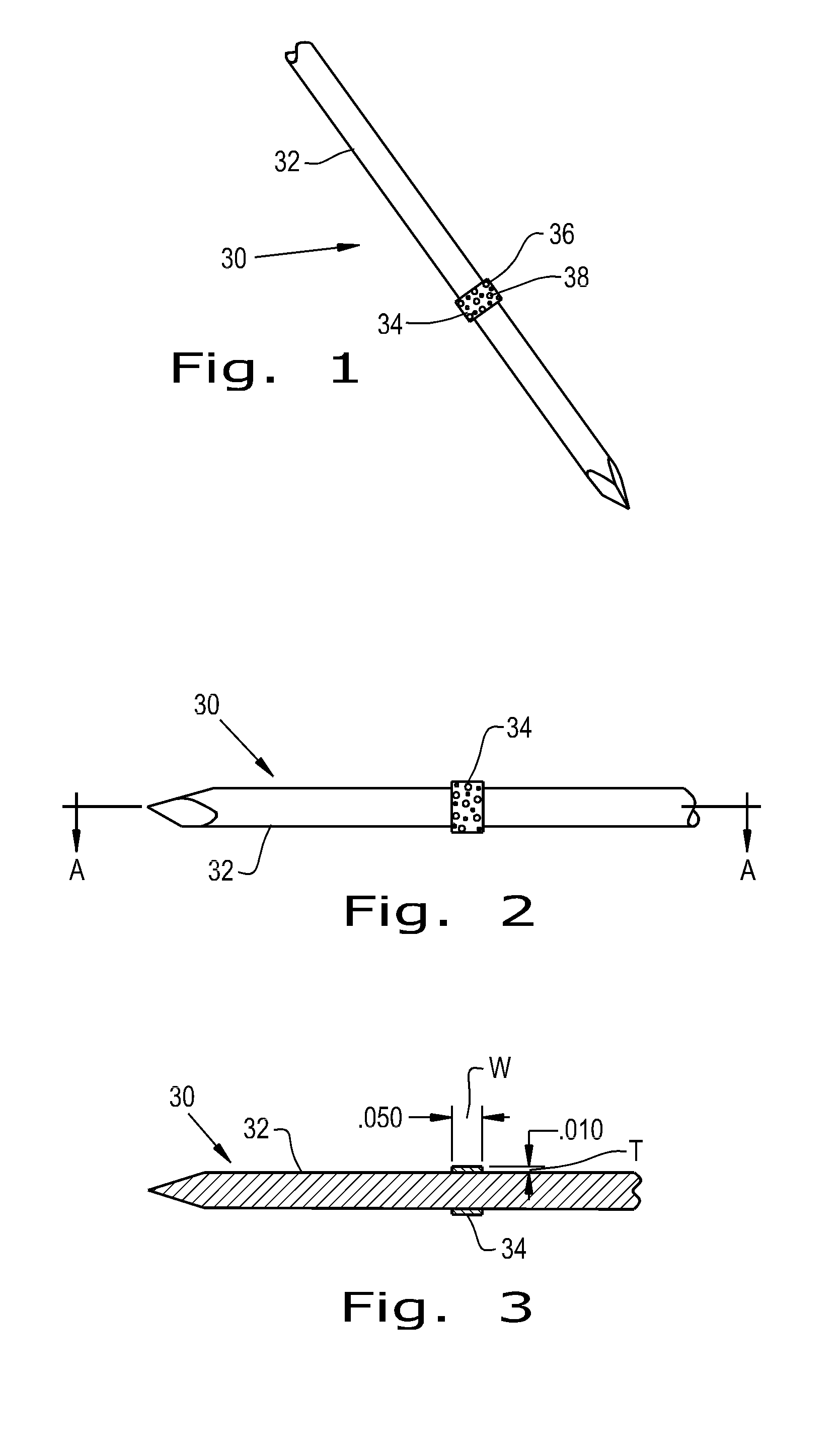
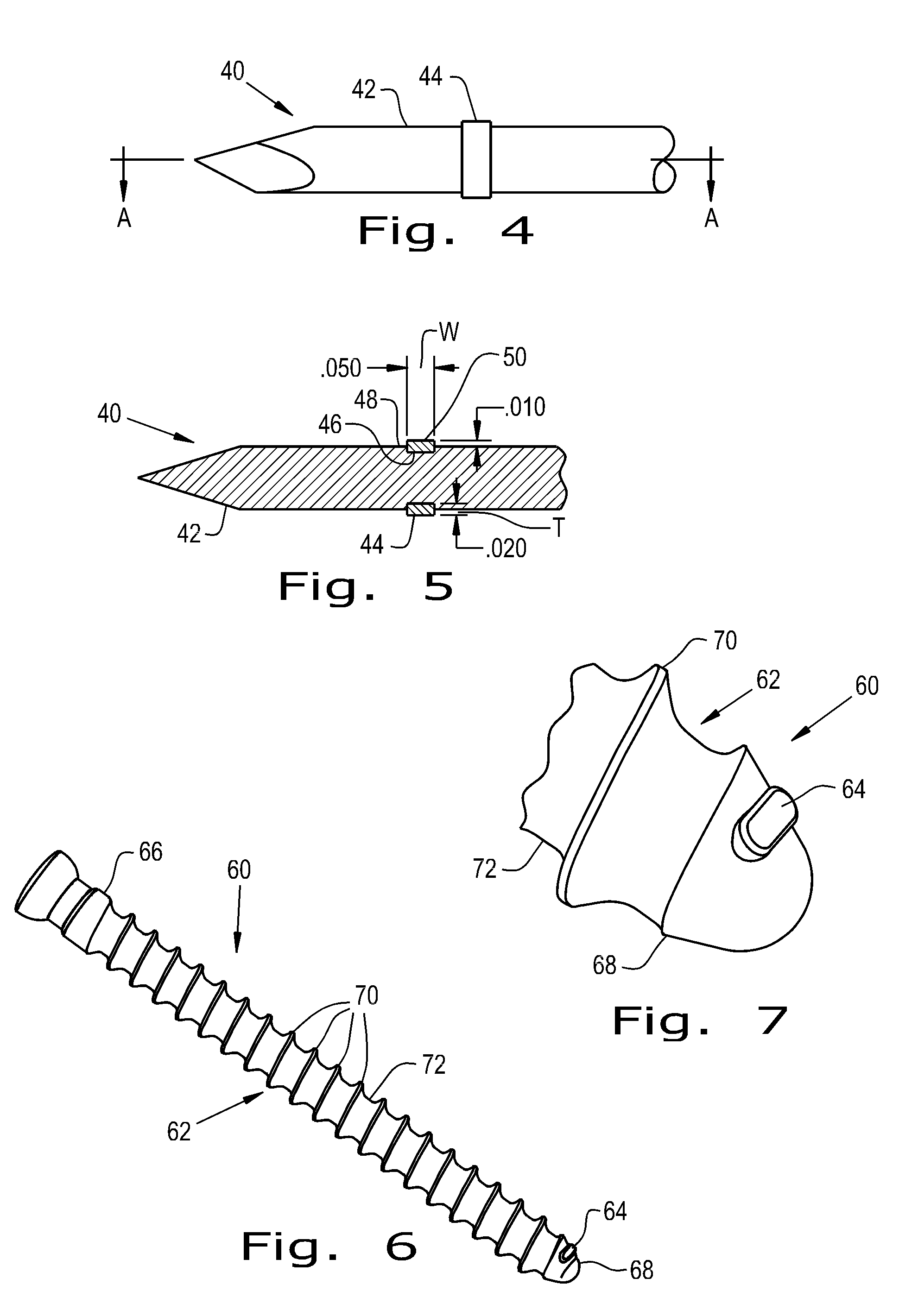
[0040]Referring now to the drawings, and more particularly to FIG. 1, there is shown an orthopaedic implant 30 which generally includes a base device 32 and a fixation material 34 attached to the base device 32. The base device 32 shown is a bone pin that can reside within a patient for a short period of time. The base device 32 can be constructed of metals commonly used in orthopaedic implants such as titanium, cobalt chrome and stainless steel. Alternatively, the base device can be constructed of biocompatible polymers such as polyether ether ketone (PEEK), polylactic acid (PLA), polyglycolic acid (PGA), polyethylene (PE) and blends thereof.
[0041]The fixation material 34 attached to the base device 32 is shaped as a thin band wrapped around the circumference of the base device 32. The fixation material 34 can be a porous polymer or metal that has a roughened surface 36 to provide immediate fixation of the device 30 due to frictional forces and to encourage quick tissue ingrowth in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| adhesive force | aaaaa | aaaaa |
| plurality of pores | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com