Formulation and method for increasing oral bioavailability of drugs
a technology of oral bioavailability and drug bioavailability, which is applied in the direction of biocide, plant/algae/fungi/lichens ingredients, biochemicals, etc., can solve the problems of poor oral bioavailability, many drugs are prone to poor oral bioavailability, so as to improve oral bioavailability, improve oral bioavailability, and improve oral bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
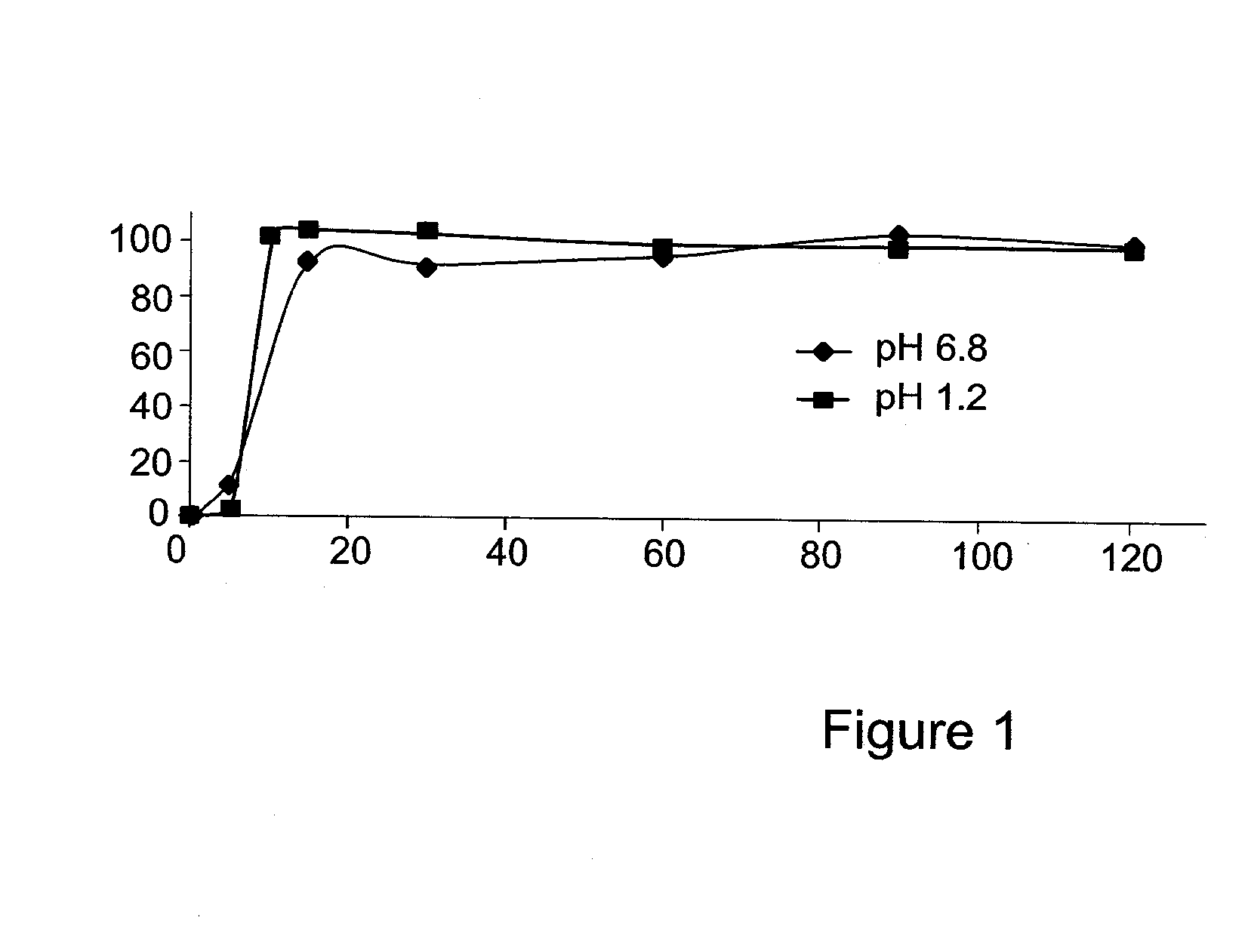
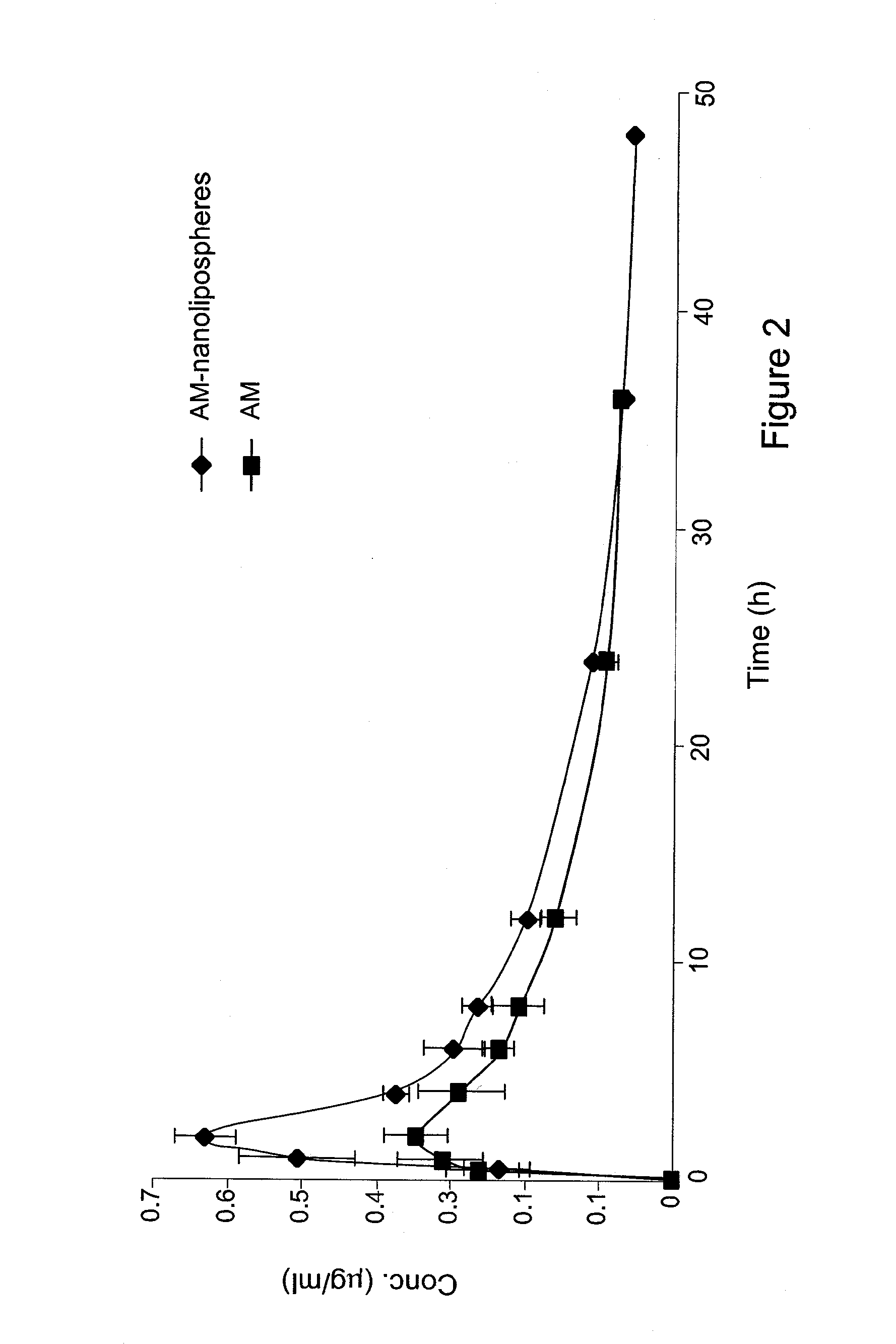
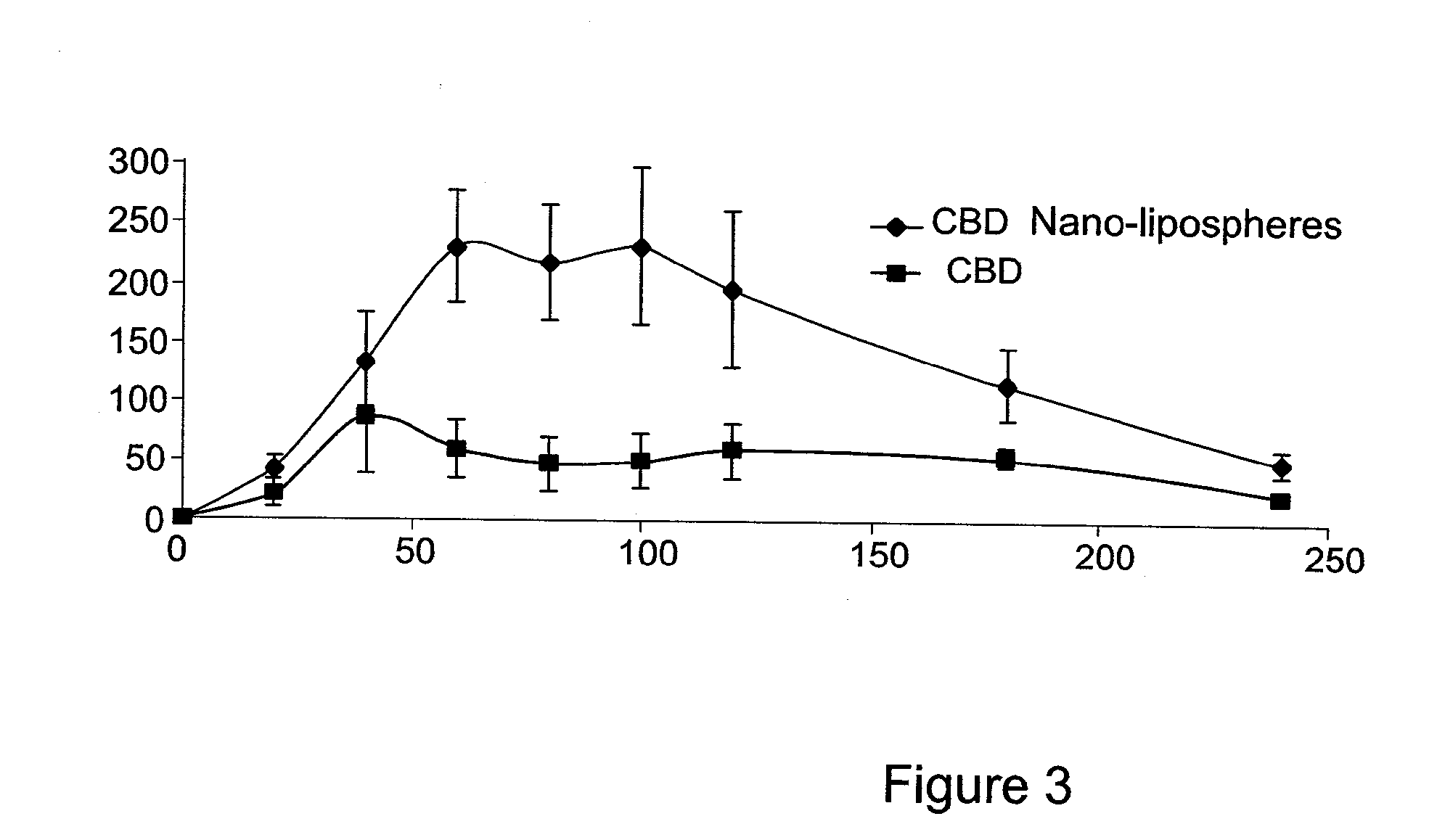
[0162]The present invention is thus based on the realization that it is possible to increase the oral bioavailability of a drug, such as a drug containing a lipophilic compound, by administering the drug in a composition comprising pro-nano lipospheres incorporating piperine.
[0163]The following specific examples illustrate various aspects of the present invention, and are not intended to be limiting in any way. For all examples, all the ingredients were dissolved in the solvent using magnetic stirrer, and heated gently (˜40-45° C.) for 15-45 minutes until the ingredients were completely dissolved. To obtain particles, the concentrate formulation was diluted in at least 10 volumes of water in aqueous solution.
[0164]For all experiments described below, the particle size of the diluted formulation was measured with VLA particle size analyzer (Coulter N4 MD Submicron Particle Size Analyzer), suitable for submicron particle size determination. About 75 μl of the concentrate formulation w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Nanoscale particle size | aaaaa | aaaaa |
| Amphiphilic | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com