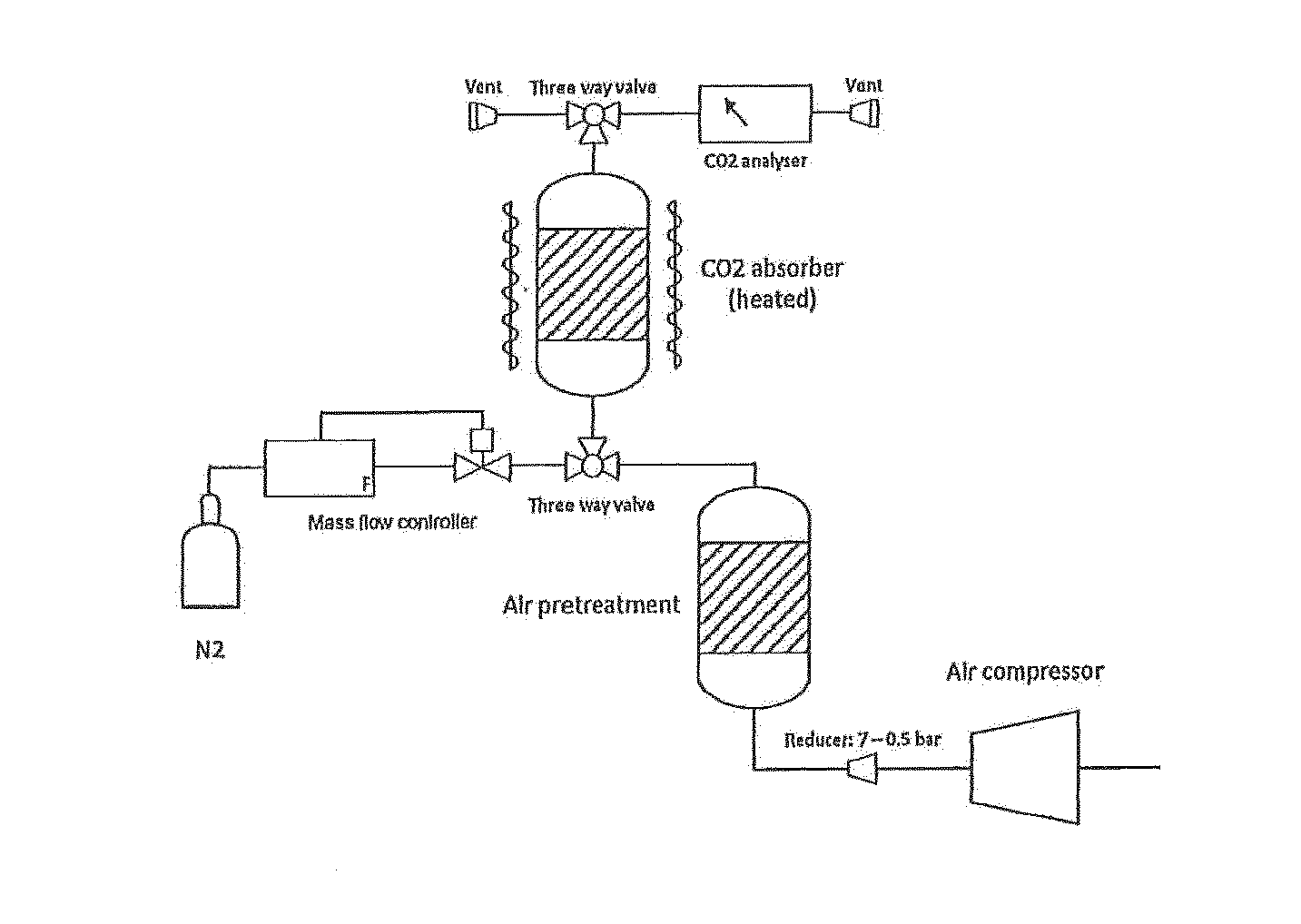
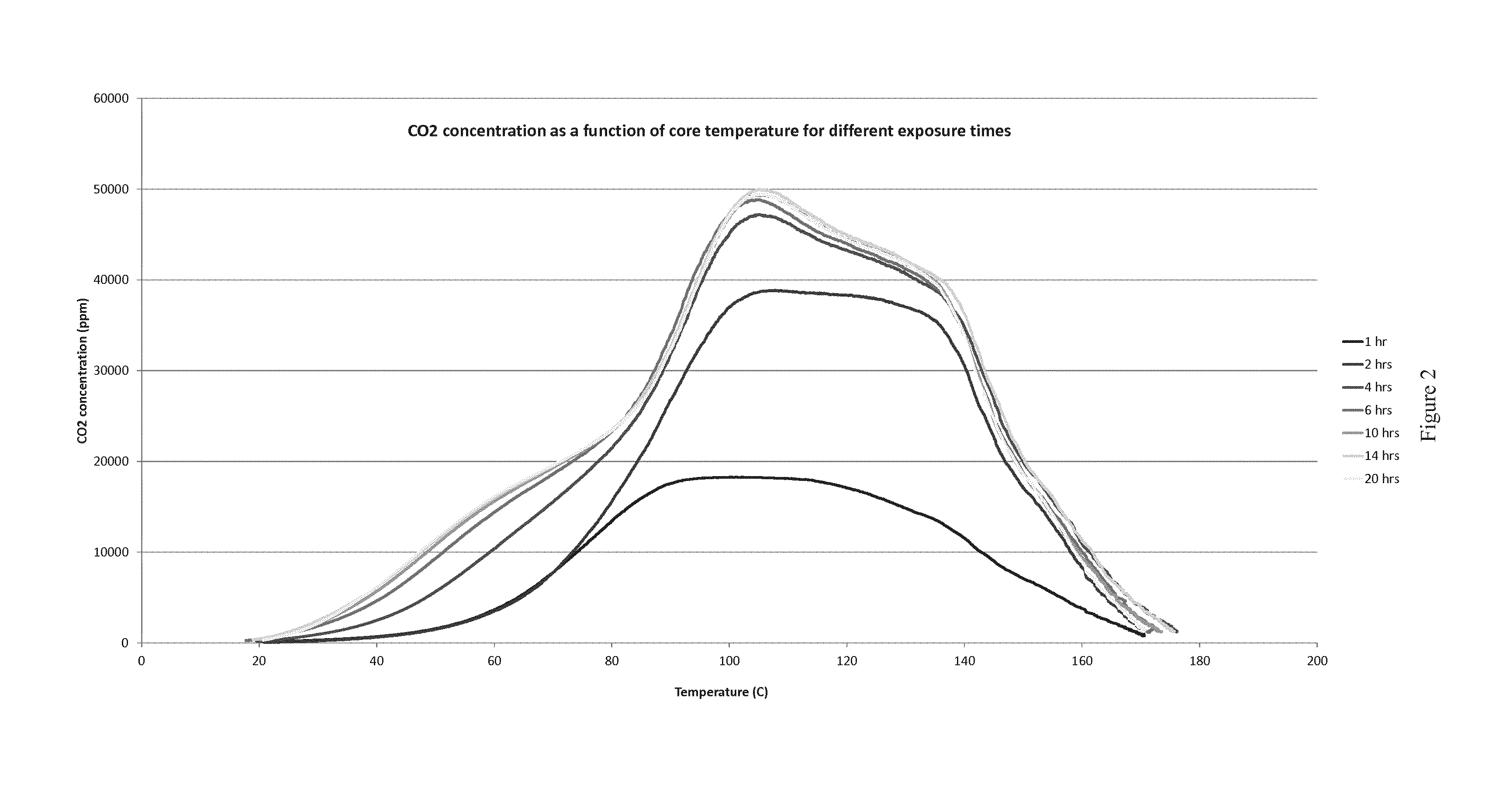
Materials and process for reversible adsorption of carbon dioxide
a technology of carbon dioxide and materials, applied in the field of reversible adsorption of carbon dioxide, can solve the problems of high temperature and considered irreversibl
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0017]The following is a detailed description of the invention.
Definition
[0018]The term “reversibly adsorbing carbon dioxide” as used herein means adsorption of carbon dioxide to a material that releases the adsorbed carbon dioxide at a temperature below 200° C.
[0019]In its broadest aspect the present invention relates to a solid material capable of reversibly adsorbing carbon dioxide, said solid material comprising a porous carrier material having deposited thereon: (i) a salt capable of reacting with carbon dioxide; and optionally (ii) a particulate, water-insoluble inorganic material.
[0020]The temperatures of adsorption and desorption are very important for the economics of the process. The solid material is preferably used in a temperature swing reaction. Large temperature swings require large energy inputs. In addition, large temperature swings tend to cause deterioration of adsorbent materials. Preferably the solid material is capable of adsorbing carbon dioxide at a first tem...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature T2 | aaaaa | aaaaa |
| temperature T2 | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com