Digital lspr for enhanced assay sensitivity
a digital lspr and assay technology, applied in the field of enhanced assay sensitivity, can solve the problems of insufficient detection of analytes in samples in minute quantities, and requires extremely sensitive assays for detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0086]Red Channel changes in response to a bulk refractive index change
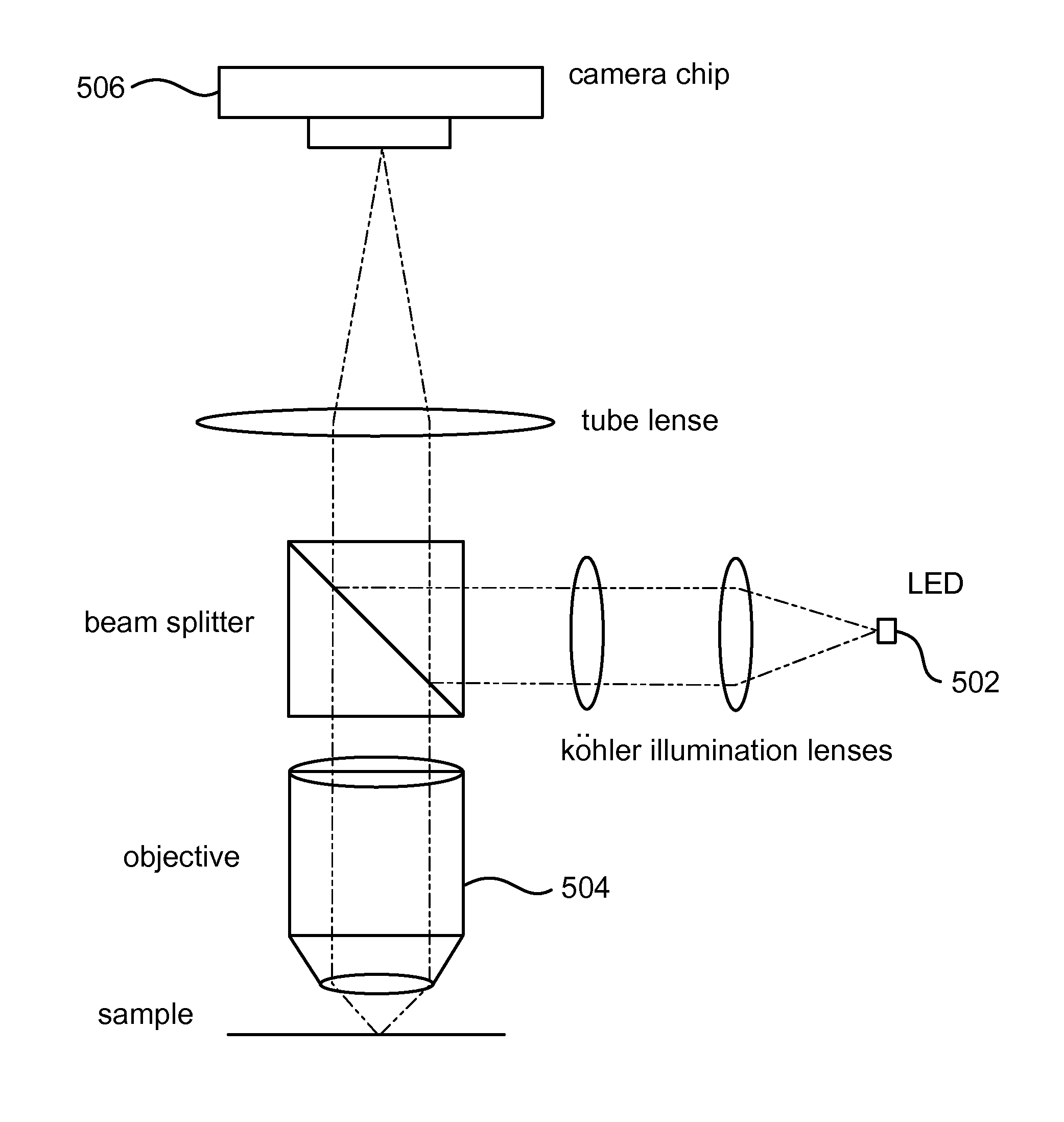
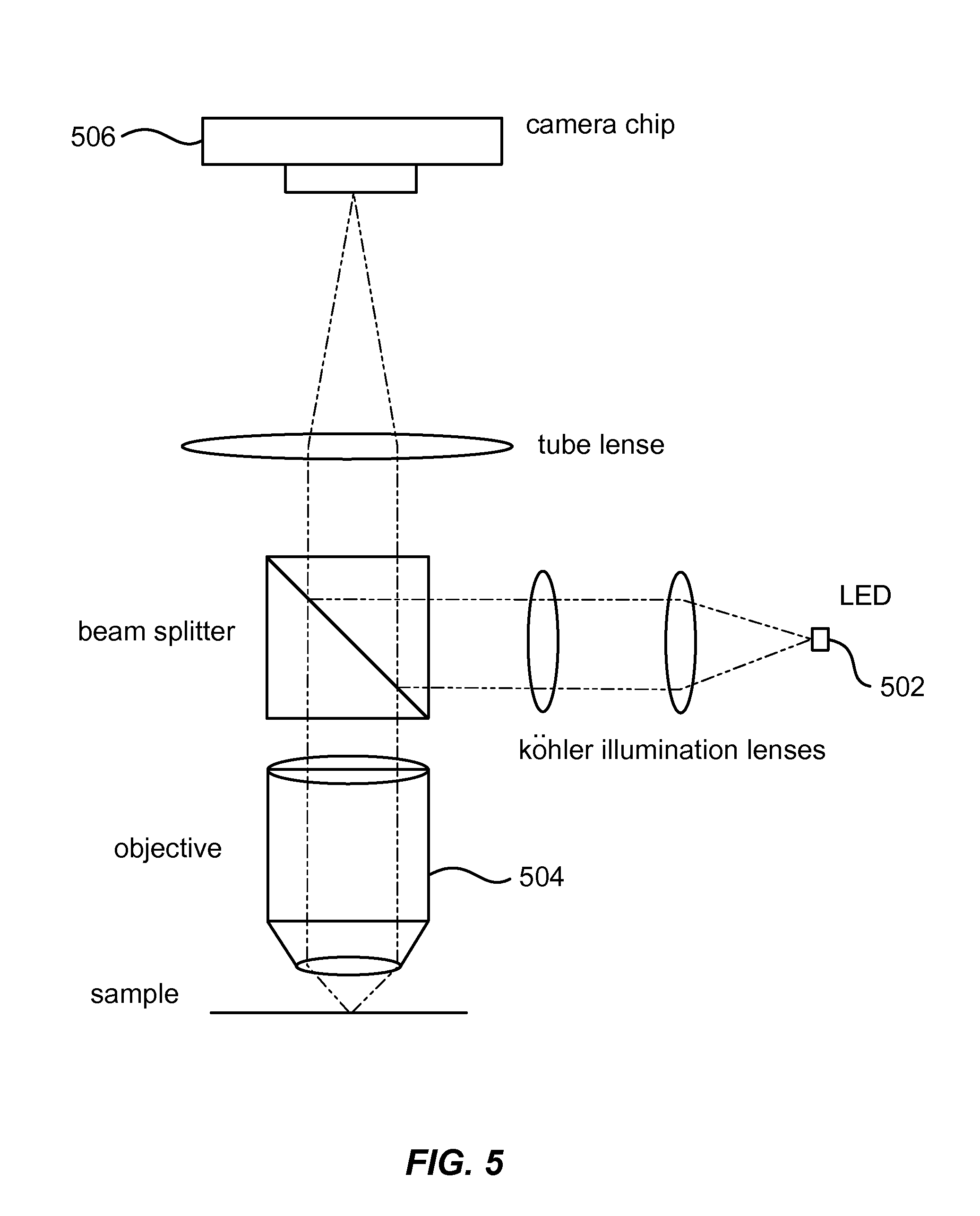
[0087]LSPR sensors were imaged using a ThorLabs CMOS camera (DCC1645C-HQ) with a 10× magnification objective placed ˜7 mm from the surface (and ˜3-4 mm from the top of a flow cell cover). In the flow cell, different solutions were pumped successively (water, 5% EG, 10% EG, and 20% EG). For each solution, a series of images were acquired and analyzed. For each series of images, pixels were selected at random and 3×3 pixels centered on the randomly selected pixel were analyzed for changes in red channel values. The sensor surface raw data collected from a color CMOS sensor shows how the red channel changes for 3×3 pixels subsection of the entire image when the bulk refractive index is successively changed from water to 5% Ethylene Glycol (EG), 10% EG, and 20% EG (shown in FIG. 11). Panel A shows a raw signal for one randomly selected pixel, while panel B shows the same signal run through an averaging filter to remo...
example 2
Limit of Detection Determination Based on Detection of Analytes at Various Surface Coverage
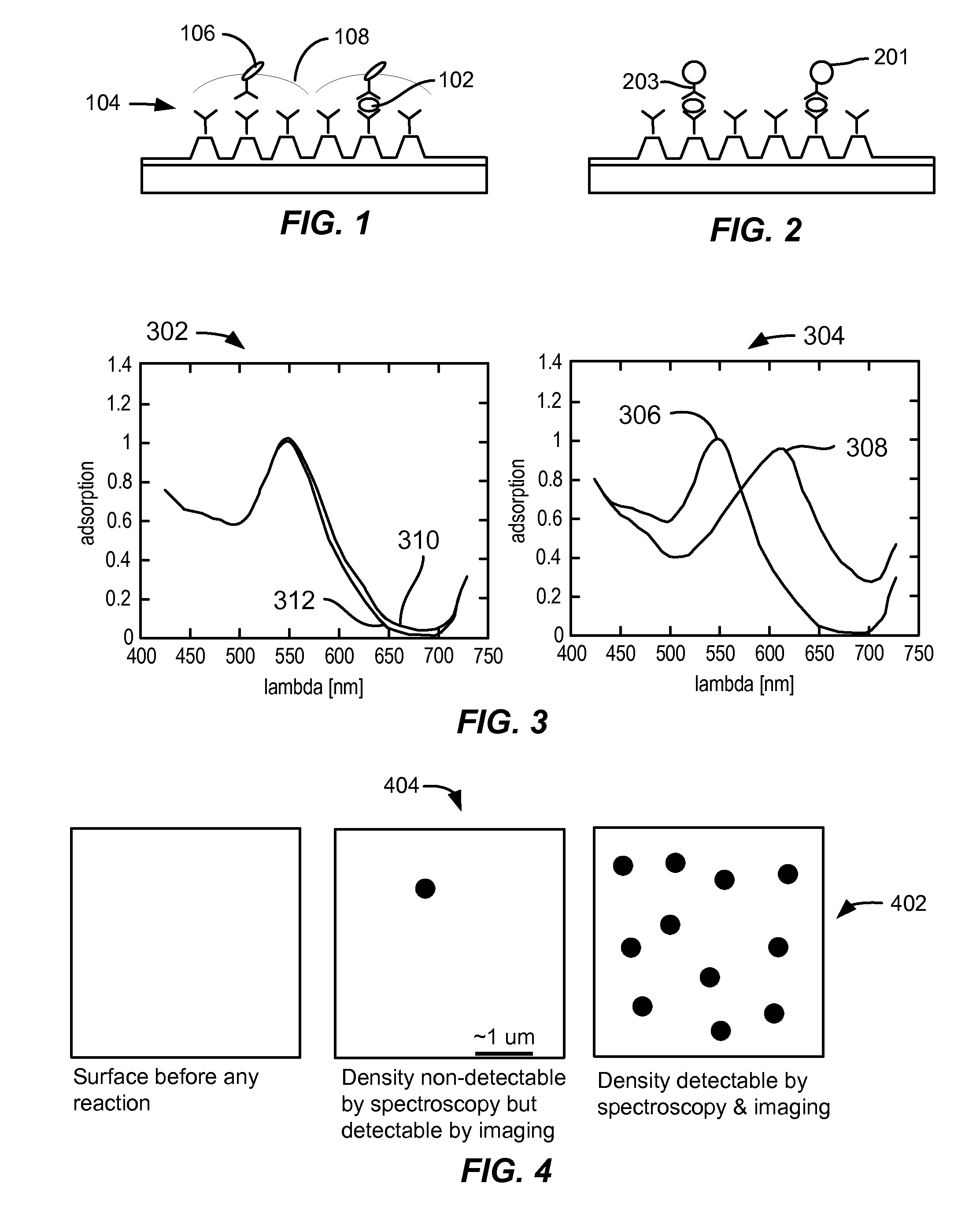
[0090]FIG. 9 illustrates the detection of analytes at several surface coverages using digital LSPR, in accordance with embodiments. In a proof of concept experiment, a surface was prepared by co-adsorbing biotinylated IgG and native IgG at various ratios, but at a fixed total antibody concentration of 1 mg / mL (biotinylated IgG plus native IgG). Ratios of 10−6, 3*10−6, 8*10−6, 10−5, 10−4, and 10−3 were specifically prepared. The total concentration was large enough to saturate the surface. The surface coverage in biotinylated IgG was assumed to correspond to the ratio of biotinylated IgG to total antibody. Hence a surface coverage of 10−6 meant that during co-adsorption of biotinylated IgG and native IgG there were 1 biotinylated IgG per 106 native IgG. These model surfaces mimic the coverage of antigen in real assays. The biotinylated antibodies on the LSPR surface was detected using streptavi...
example 3
Prophetic
Detection of Nucleic Acid Target Molecules
[0092]In some embodiments, the disclosed digital LSPR methods and systems may be used for detection of target nucleic acid sequences in biological or environmental samples. One of skill in the art can envision immobilizing capture probes on the LSPR sensor surface that are complementary to a portion of one or more target nucleic acid sequences. Immobilization of probes on the surface may be accomplished by any of a variety of techniques known to those of skill in the art, for example by using patterned, self-assembled monolayers of thiolated oligonucleotides (or suitable linker molecules, as necessary) on noble metal surfaces. The surface may then be contacted with a sample of interest under conditions that promote hybridization of complementary sequences such that target nucleic acid molecules, if present, are captured and immobilized on the sensor surface. The sensor surface may then be rinsed and contacted with a solution compris...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com