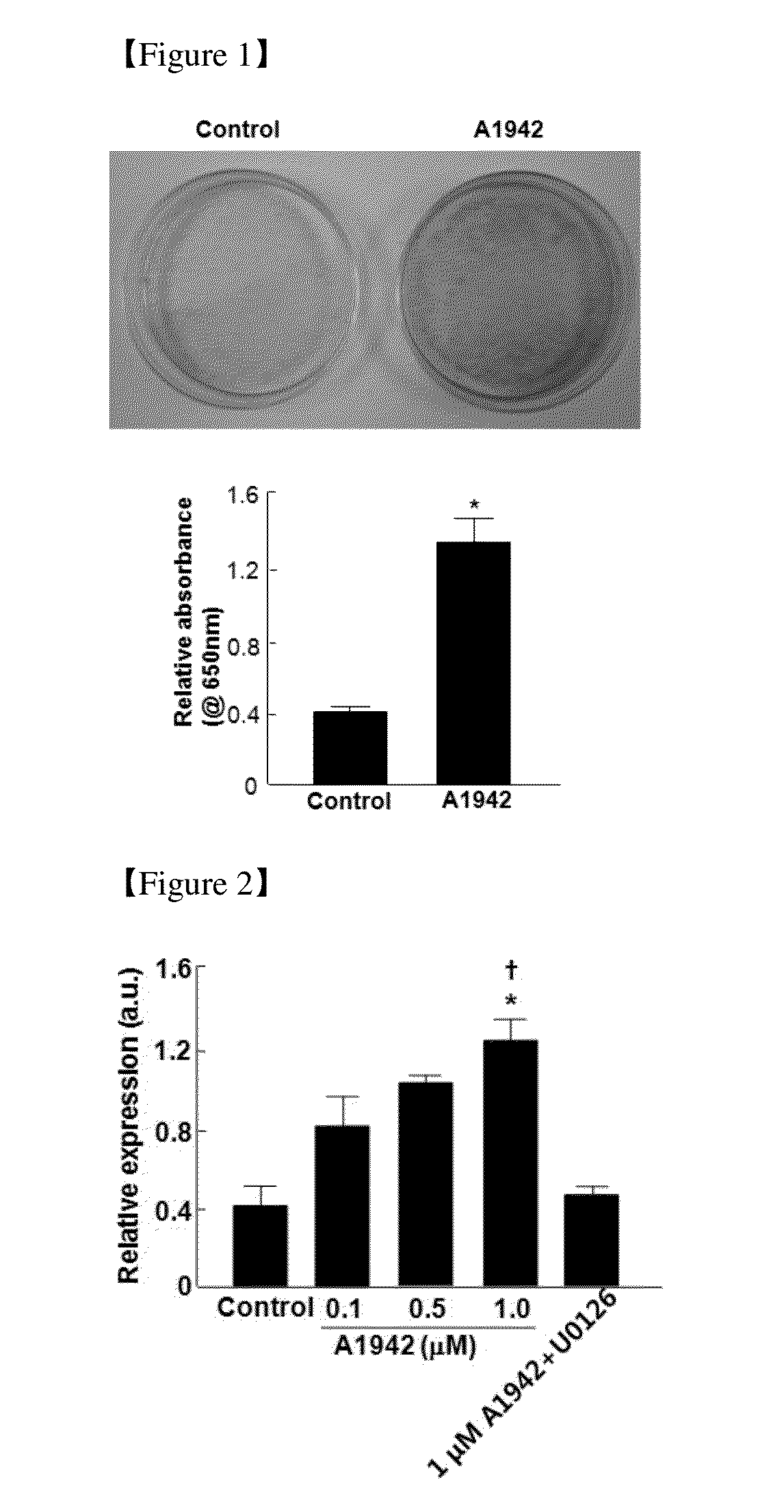
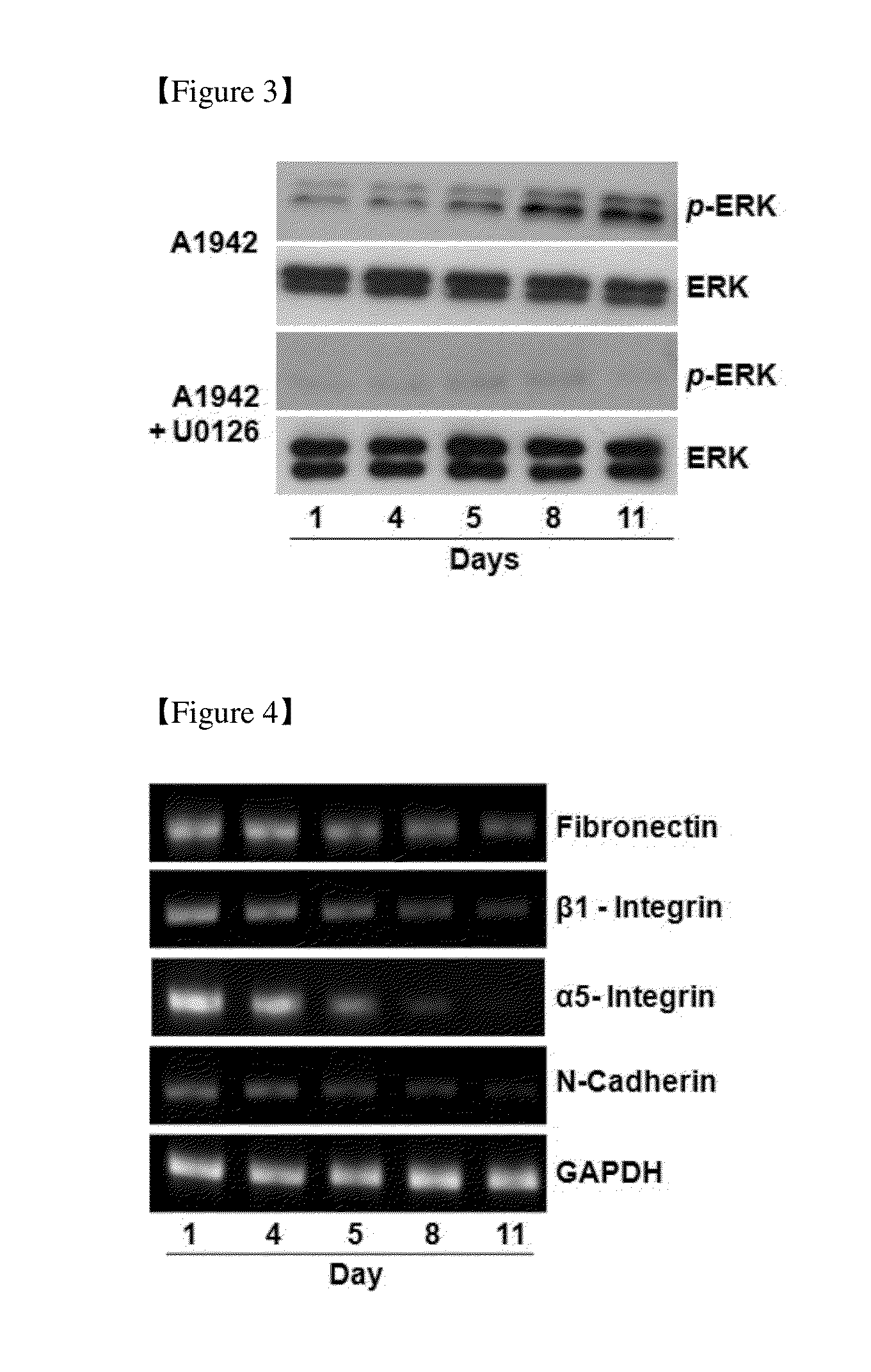
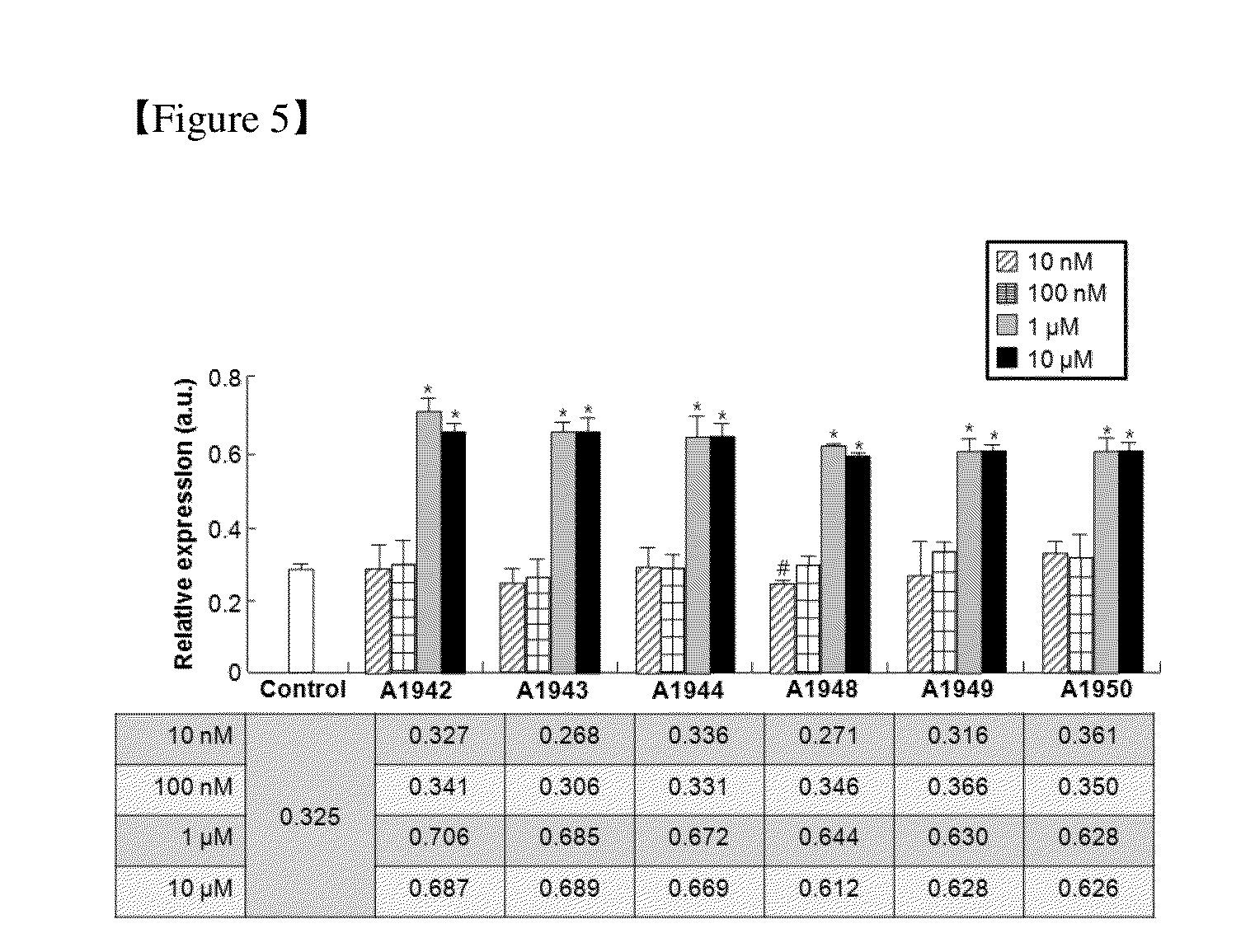
Use of a Compound for Inducing Differentiation of Mesenchymal Stem Cells into Cartilage Cells
a technology of mesenchymal stem cells and compounds, which is applied in the direction of heterocyclic compound active ingredients, drug compositions, skeletal/connective tissue cells, etc., can solve the problems of non-specifically relieving pain or an inflammatory response, affecting the regeneration or proliferation of chondrocytes, so as to achieve the effect of treating cartilage diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
Synthesis of Compound of Formula 1
Example 1
Preparation of (quinoline-8-sulfonic acid (4-hydroxy-phenyl)-amide
[0086]
[0087]The compound of Formula 3 (216 mg, 1.98 mmol) and triethylamine (550 ml, 3.96 mmol) were introduced into a 0.2 M tetrahydrofuran solution including the compound of Formula 2 (300 mg, 1.32 mmol) while stirring, and stirred for 12 hours. The resulting mixture was filtered with ethylacetate, and then concentrated under a reduced pressure. The obtained primary compound was purified using silica gel column chromatography (eluent: ethylacetate:methylene chloride:hexane=2:1:3) to obtain a title compound at a yield of 49% (194 mg).
[0088]1H-NMR (500 MHz, DMSO-d6) δ 9.46 (s, 1H), 9.19 (s, 1H), 9.15 (dd, J=4.2, 1.8 Hz, 1H), 8.53 (dd, J=8.4, 1.7 Hz, 1H), 8.25 (dd, J=8.2, 1.4 Hz, 1H), 8.21 (dd, J=7.3, 1.4 Hz, 1H), 7.74 (dd, J=8.3, 4.2 Hz, 1H), 7.68-7.63 (m, 1H), 6.75-6.70 (m, 2H), 6.53-6.41 (m, 2H)
examples 2 to 28
[0089]Compounds of Examples 2 to 28 were prepared in the same manner as in the preparation method described in Example 1. Chemical structures and physical properties of the prepared compounds are listed in the following Table 1 and Table 2.
TABLE 1CompoundIUPACExamplesNumberChemical StructureFormulaNomenclature 1A1942C15H12N2O3SQuinoline-8- sulfonic acid (4- hydroxy-phenyl)- amide 2A1943C19H14N2O3SQuinoline-8- sulfonic acid (7- hydroxy- naphthalen-1-yl)- amide 3A1944C16H12N2O5S2-Hydroxy-5- (quinoline-8- sulfonylamino)- benzoic acid 4A1948C17H19NO5S5-(4-Tert-butyl- benzenesulfonyl- amino)-2-hydroxy- benzoic acid 5A1949C22H17NO3SBiphenyl-4- sulfonic acid (5- hydroxy- naphthalen-1-yl)- amide 6A1950C17H15NO3SN-(4-hydroxy- naphthalen-1-yl)- 4-methyl- benzenesulfonamide 7A1880C16H13NO3SNaphthalene-2- sulfonic acid (4- hydroxyl- phenyl)-amide 8A1881C17H15NO3SN-(5-hydroxyl- naphthalen-1-yl)- 4-methyl- benzenesulfonamide 9A1882C17H15NO3SN-(7-hydroxyl- naphthalen-1-yl)- 4-methyl- benzenesulfon...
experimental example 1
Confirmation of Differentiation of Mesenchymal Stem Cells to Chondrocytes by Compound of Formula 1
[0090]Separation and Incubation of Human Fat-Derived Mesenchymal Stem Cells
[0091]60 cc of an expanding solution (obtained by adding 30 mL of 1% lidocaine, 30 mL of 0.5% bupivacaine, 10 mL of 4.2% sodium bicarbonate and 1 mg of epinephrine to 1 L of normal water for injection) was applied to the periphery of a navel under sterile conditions. Fats were suctioned using Mercedes 3 mm×9 cm Aspiration Luer Lock Cannula (Byron Medical, Tucson, Ariz.), and then instantly kept on ice. Since a lipoaspirate filtered through a 250 mm sieve included an anesthetic and a contaminant such as blood, the lipoaspirate was washed with phosphate buffered saline (PBS) to remove the anesthetic and the contaminant. Collagenase I (1 mg / mL; Worthington Biochemical Corp., Lakewood, N.J.) was added to the obtained fat tissues, stirred, and then kept at 37° C. for an hour. The suspended adipose tissues were centrif...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com