Room temperature polymer crosslinking using 1-functionalized benzocyclobutene
a technology of benzocyclobutene and polymer, applied in the field of benzocyclobutene (bcb) monomers, can solve the problems of limiting the use of ring openings in many systems, requiring very high temperatures (>200° c), and ignoring the activation of bcbs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
Introduction
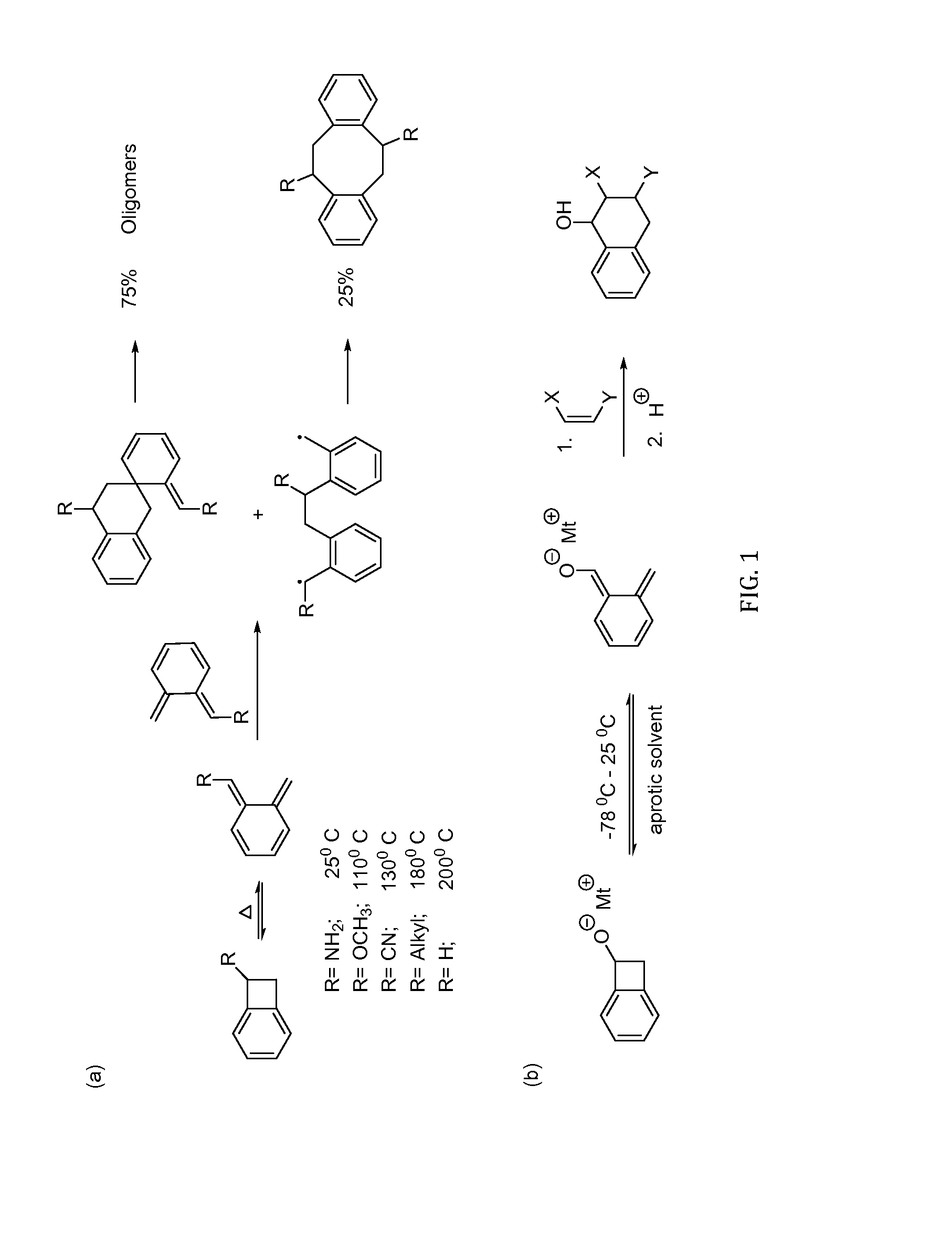
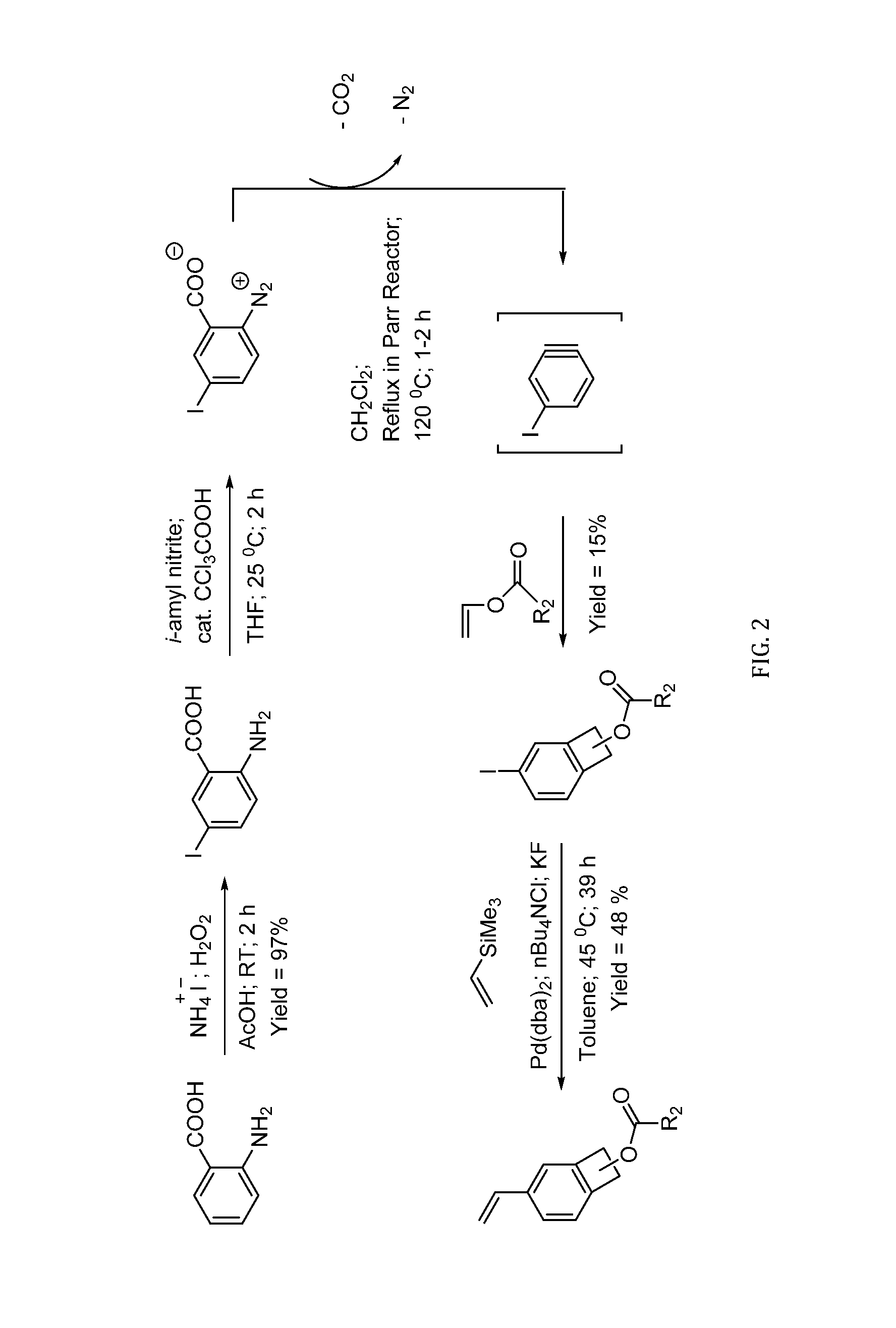
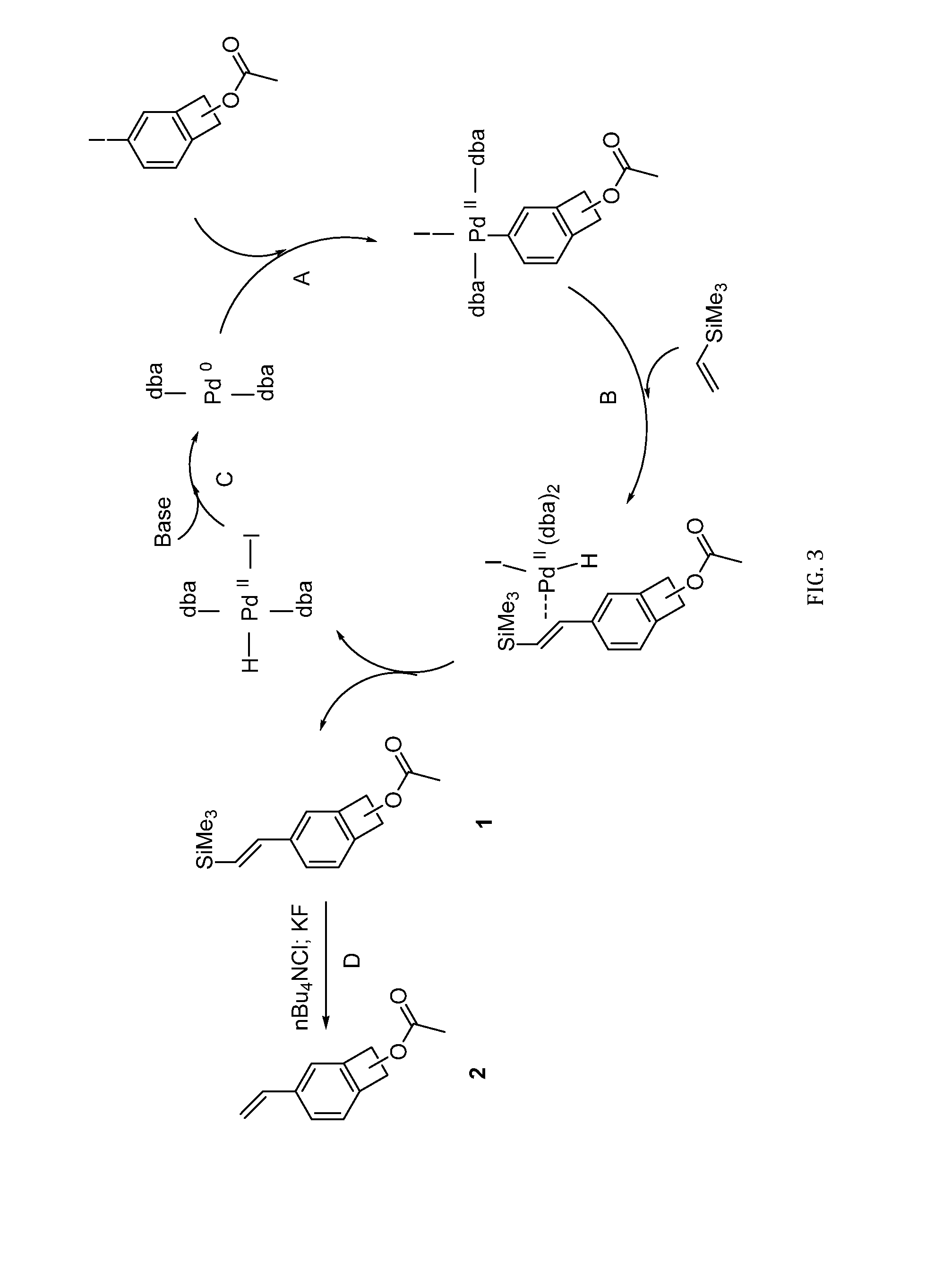
[0048]Prior work reported the synthesis of a new monomer based on 1-functionalized vinyl BCB that contains a vinyl group on the aromatic ring and a substituent on the benzylic position. See Pugh, C.; Baker, J. S.; Storms, W. K. SynLett 2014, 25, 148-152, “Synthesis of a Polymerizable Benzocyclobutene that Undergoes Ring-Opening Isomerization at Reduced Temperature.” This monomer could advantageously be incorporated into a polymer backbone via the vinyl group and could be crosslinked post-polymerization using the BCB group. However the temperature for crosslinking was in the range of from 100-150° C., and lower crosslinking temperatures would be preferred. Thus, a specific embodiment of this invention provides a new, ambient temperature crosslinker on the same lines; ideally making co-1-alkoxide-4 / 5-vinylBCB. However, it was found the higher reactivity of 1-alkoxideBCB towards dienophiles at lower temperatures would make co-1-alkoxide-4 / 5-vinylBCB highly unstable and diff...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com