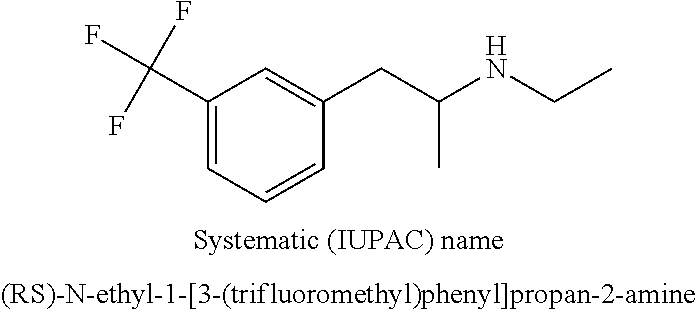
Methods of treating lennox-gastaut syndrome using fenfluramine
a technology of fenfluramine and lennox-gastaut syndrome, which is applied in the field of human patient treatment, can solve the problems of withdrawn from the us and global market, sorely needed treatment options, and the efficacy of fenfluramin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Add-On Therapy with Low Dose Fenfluramine (FFA) in Lennox-Gastaut
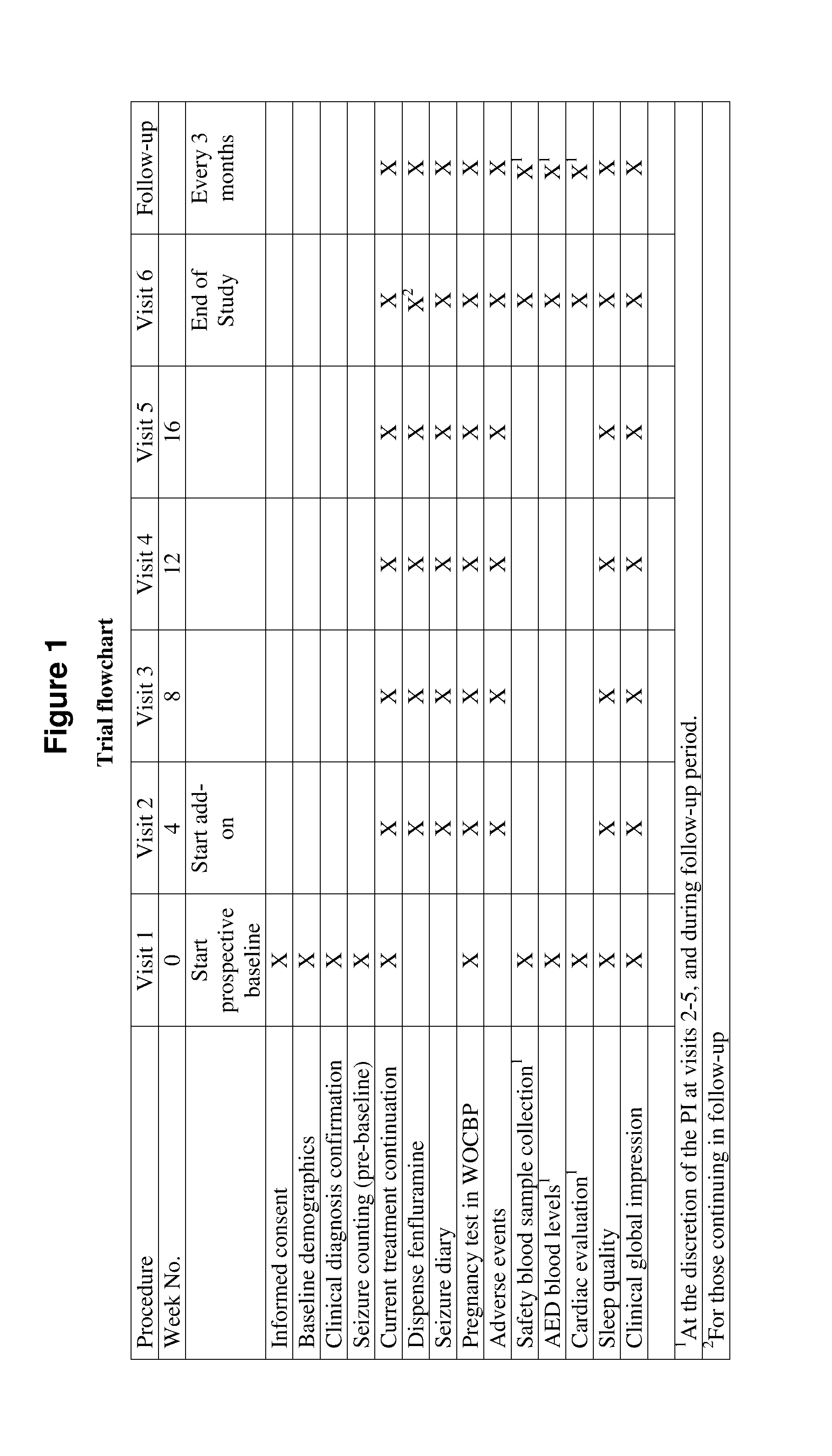
[0145]The efficacy of fenfluramine as an add-on treatments in Lennox-Gastaut patients is studied in a Phase 2 Clinical Trial. The study protocol is described and preliminary results are presented here.
Trial Objectives and Design
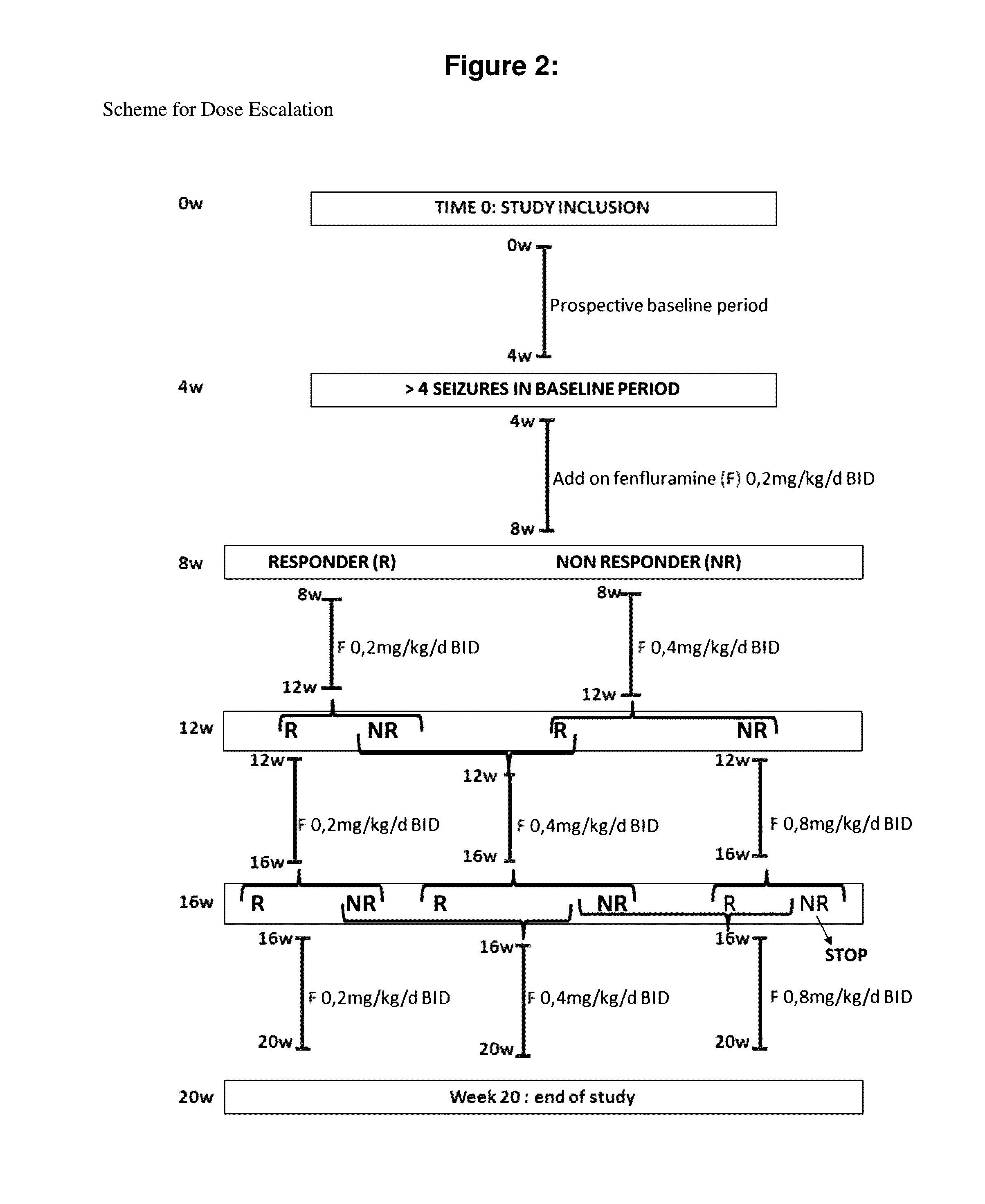
[0146]An open-label, non-placebo controlled add-on study was designed to assess the efficacy and safety of low-dose add-on fenfluramine across a range of fenfluramine doses (0.2, 0.4, 0.8 mg / kg / day, to a maximum of 30 mg / day). The trial was conducted over a 20 week period, with responders eligible for follow-on treatment, with follow-up appointments at three month intervals.
Inclusion and Exclusion Criteria
[0147]Patients were recruited from childhood epilepsy clinics in Leuven and Antwerp, Belgium, and selected for inclusion in the study according to criteria comprising a combination of age, physical and psychological characteristics, and resistance to treatment with conventional therapies. Detai...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
| total seizure frequency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com