Cryogenic System and Method of Use
a cryogenic system and cryogenic technology, applied in the field of cryogenic systems, can solve the problems of tissue freezing, inability to operate or withstand pressures greater than 500 psi, use of heat exchangers, etc., and achieve the effects of enhancing nucleation and deposition of saturated gas, enhancing deposition or nucleation modification, and facilitating cooling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0025]In the following detailed description, for purposes of explanation and not limitation, exemplary embodiments disclosing specific details are set forth in order to provide a thorough understanding of the present invention. However, it will be apparent to one having ordinary skill in the art that the present invention may be practiced in other embodiments that depart from the specific details disclosed herein. In other instances, detailed descriptions of well-known devices and methods may be omitted so as not to obscure the description of the present invention.
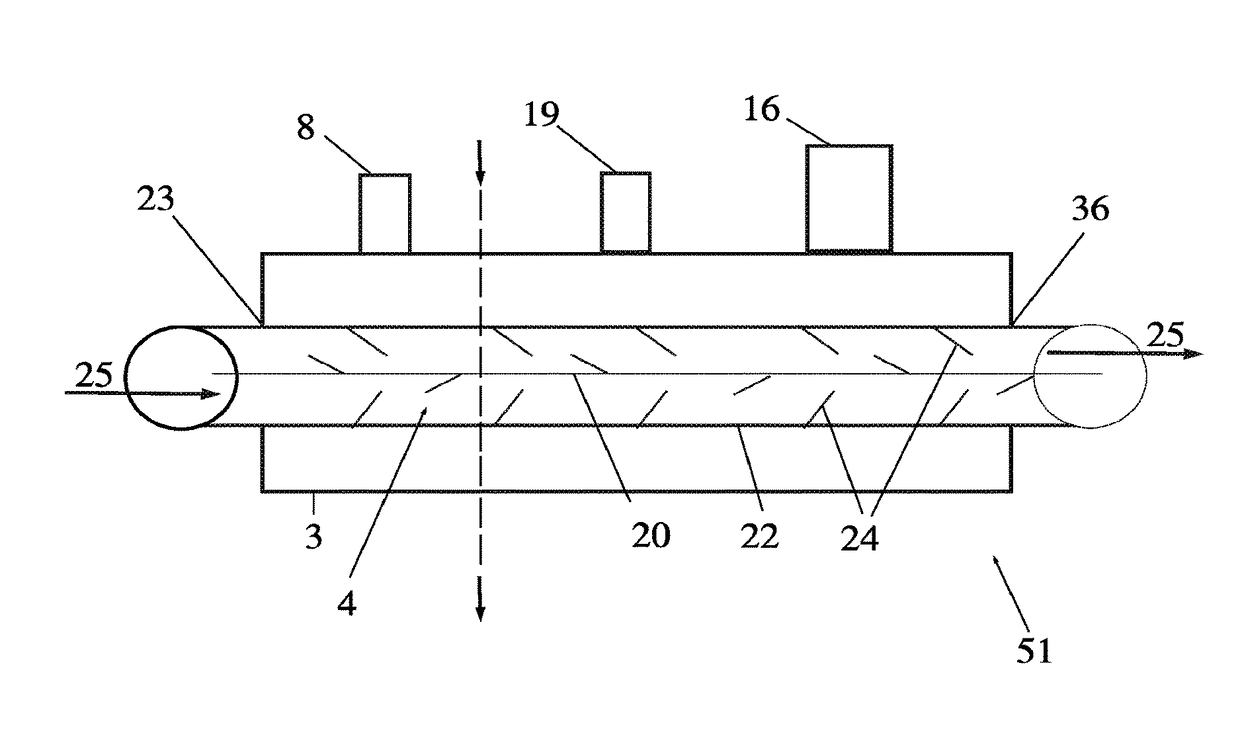
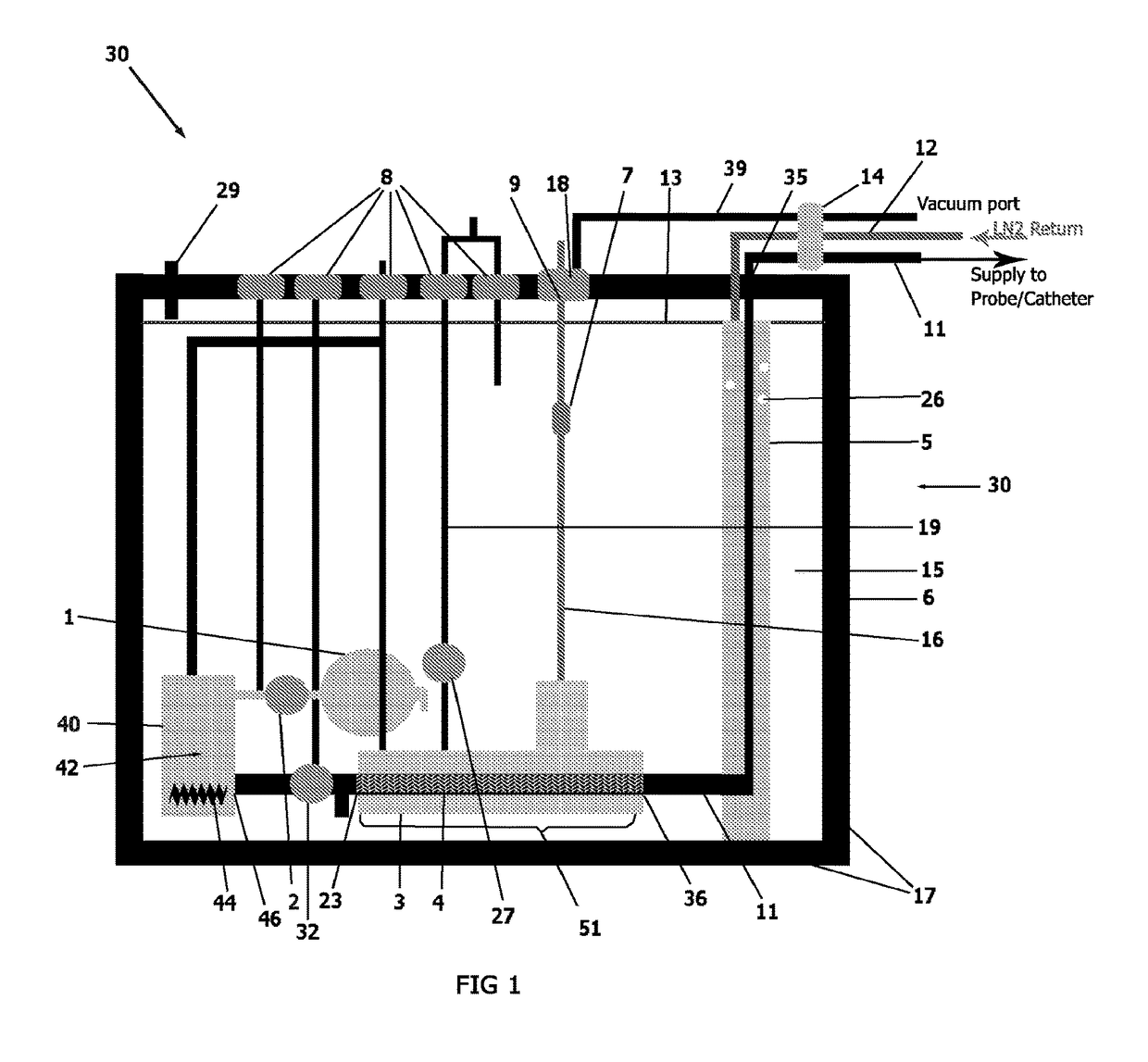
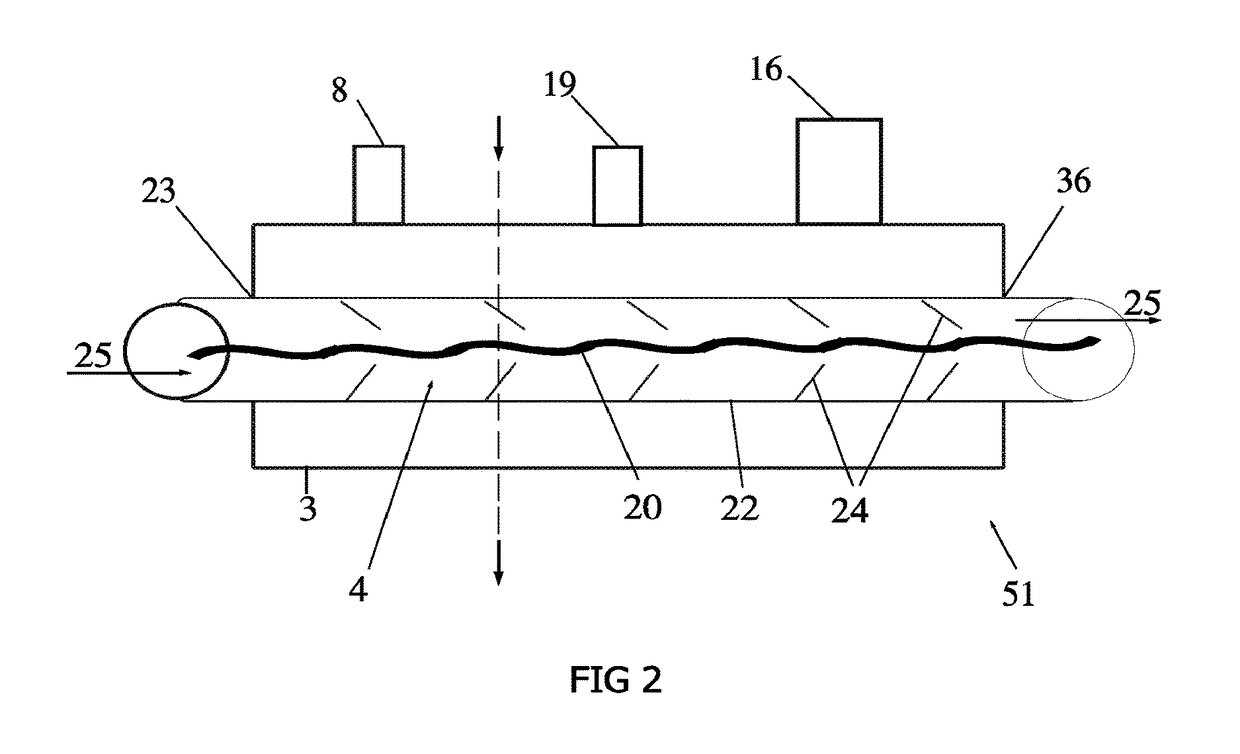
[0026]An external view of a device and system in accordance with one embodiment of the present invention is shown in FIG. 1. The cryogenic system or device 30 has sidewalls 17 which form a container 6 that encloses an internal cavity, or lumen 15. In an embodiment of FIG. 1, the container 6 takes the form of a vacuum insulated dewar 6. The dewar 6 stores liquid cryogen and interconnects a supply line 11 and return line 12 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com