Bisphenol derivatives and their use as androgen receptor activity modulators
a technology of androgen receptors and derivatives, applied in the field of bisphenol related compounds, can solve problems such as hammering virtual docking drug discovery approaches
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
of (R)-3-(4-(2-(3,5-dichloro-4-((S)-3-chloro-2-hydroxypropoxy)phenyl)propan-2-yl)phenoxy)propane-1,2-diol (Compound 1a)
[0271]
Steps a and b: Synthesis of (S)-4-(2-(4-((2,2-dimethyl-1,3-dioxolan-4-yl)methoxy)phenyl)propan-2-yl)phenol
[0272]The titled compound was synthesized as previously reported. See, WO 2014 / 179867.
Step c: (S)-2,6-dichloro-4-(2-(4-((2,2-dimethyl-1,3-dioxolan-4-yl)methoxy)phenyl)propan-2-yl)phenol
[0273]To a solution of (S)-4-(2-(4-((2,2-dimethyl-1,3-dioxolan-4-yl)methoxy)phenyl)propan-2-yl)phenol (500 mg, 1.46 mmol, 1.0 equiv) in MeOH (12 mL) was added NaCl (256 mg, 4.38 mmol, 3.0 equiv) and NaOH (87.6 mg, 2.19 mmol, 1.5 equiv). Aqueous sodium hypochlorite (6035 mg, 5.4% in H2O, 4.38 mmol, 3.0 equiv) was then added dropwise over 2 min at 0° C. After 2 hours, the mixture was extracted with ethyl acetate (2×30 mL). The organic layer was washed with deionized water (2×30 mL), dried over anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure. The ...
example 2
of (R)-3-(4-(2-(3,5-dibromo-4-((S)-3-chloro-2-hydroxypropoxy)phenyl)propan-2-yl)phenoxy)propane-1,2-diol (Compound 3a)
[0276]
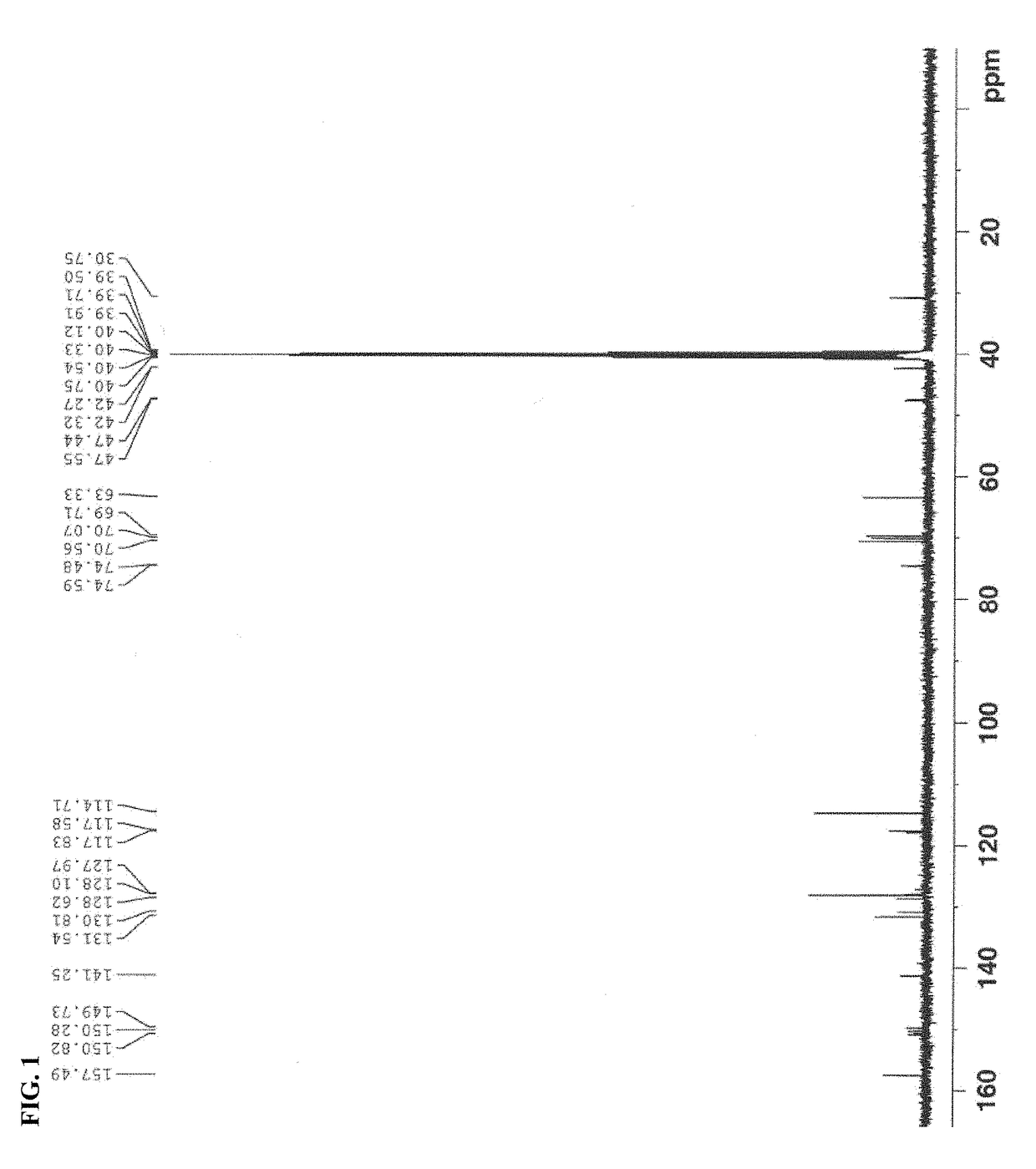
[0277]Compound 3a was synthesized according to Example 1 by suing NaBr instead of NaCl in step c. 1H NMR (400 MHz, DMSO-D6) δ (ppm)=7.39 (s, 1H), 7.30 (dd, J=2.0 Hz, 34.4 Hz, 1H), 7.15 (d, J=8.8 Hz, 2H), 6.86 (d, J=8.8 Hz, 2H), 5.57-5.54 (m, 1H), 4.91 (d, J=4.8 Hz, 1H), 4.64 (t, J=5.6 Hz, 1H), 4.10-4.08 (m, 1H), 3.98-3.92 (m, 3H), 3.86-3.81 (m, 2H), 3.79-3.76 (m, 1H), 3.71 (dd, J=5.6 Hz, 11.2 Hz, 1H), 3.45-3.42 (m, 2H), 1.60 (s, 6H). 13C NMR spectrum of Compound 3a as synthesized is shown in FIG. 1.
example 3
of (S)-1-chloro-3-(2,6-dichloro-4-(2-(4-((R)-2-hydroxy-3-methoxypropoxy)phenyl)propan-2-yl)phenoxy)propan-2-ol (Compound 5a)
[0278]
Step a: Synthesis of (S)-3-(4-(2-(3,5-dichloro-4-((S)-3-chloro-2-hydroxypropoxy)phenyl)propan-2-yl)phenoxy)-2-hydroxypropyl 4-methylbenzenesulfonate
[0279]The titled compound was synthesized by tosylation of Compound 1a under basic conditions according to commonly known protocol, such as the protocol referenced for step a in Example 1.
Step b: Synthesis of (S)-1-chloro-3-(2,6-dichloro-4-(2-(4-(((R)-oxiran-2-yl)methoxy)phenyl)propan-2-yl)phenoxy)propan-2-ol
[0280]The titled compound was synthesized via epoxidation reaction commonly known in the art under basic conditions. 1H NMR (400 MHz, CDCl3) δ (ppm)=7.13-7.10 (m, 4H), 6.86 (d, J=6.8 Hz, 2H), 4.24-4.12 (m, 4H), 3.99-3.94 (m, 1H), 3.85 (dd, J=5.2 Hz, 11.2 Hz, 1H), 3.77 (dd, J=5.6 Hz, 11.2 Hz, 1H), 3.38-3.33 (m, 1H), 2.93-2.89 (m, 1H), 2.76 (dd, J=2.4 Hz, 4.8 Hz, 1H), 1.62 (s, 6H).
Step c: Synthesis of (S)-1-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com