Method for measuring viscosity of protein solution
a protein solution and viscosity technology, applied in the direction of instruments, indirect flow property measurement, peptides, etc., can solve the problems of affecting the overall development, difficult to reduce the viscosity with absolute certainty, and the inability to perform viscosity evaluation. , to achieve the effect of reducing the viscosity and low the viscosity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[Example 1] Correlation Between Particle Size Parameters and Viscosity for Mab1, Mab2. And MAb3
[0092]MAb1: a bispecific antibody that recognizes blood coagulation factor IX and / or activated blood coagulation factor IX, and blood coagulation factor X and / or activated blood coagulation factor X. ACE910 (Q499-z121 / J327-z119 / L404-k), which is a bispecific antibody described in a non-patent document (PLoS One. 2013:8(2):e57479) and a patent document (WO 2012 / 067176) (a bispecific antibody in which the H chain comprising the amino acid sequence of SEQ ID NO: 3 and the L chain of SEQ ID NO: 5 are associated, and the H chain comprising the amino acid sequence of SEQ ID NO: 4 and the L chain of SEQ ID NO: 5 are associated), was prepared according to the description in the aforementioned non-patent document or the patent document. As described in the aforementioned patent document, ACE910 has an activity of substituting for the function of coagulation factor VIII.
[0093]MAb2: an anti-IL-6 rece...
example 2
[Example 2] Correlation of Viscosity with Particle Size Parameters and Molecular Weight Parameters for MAb1, MAb3, and MAb4
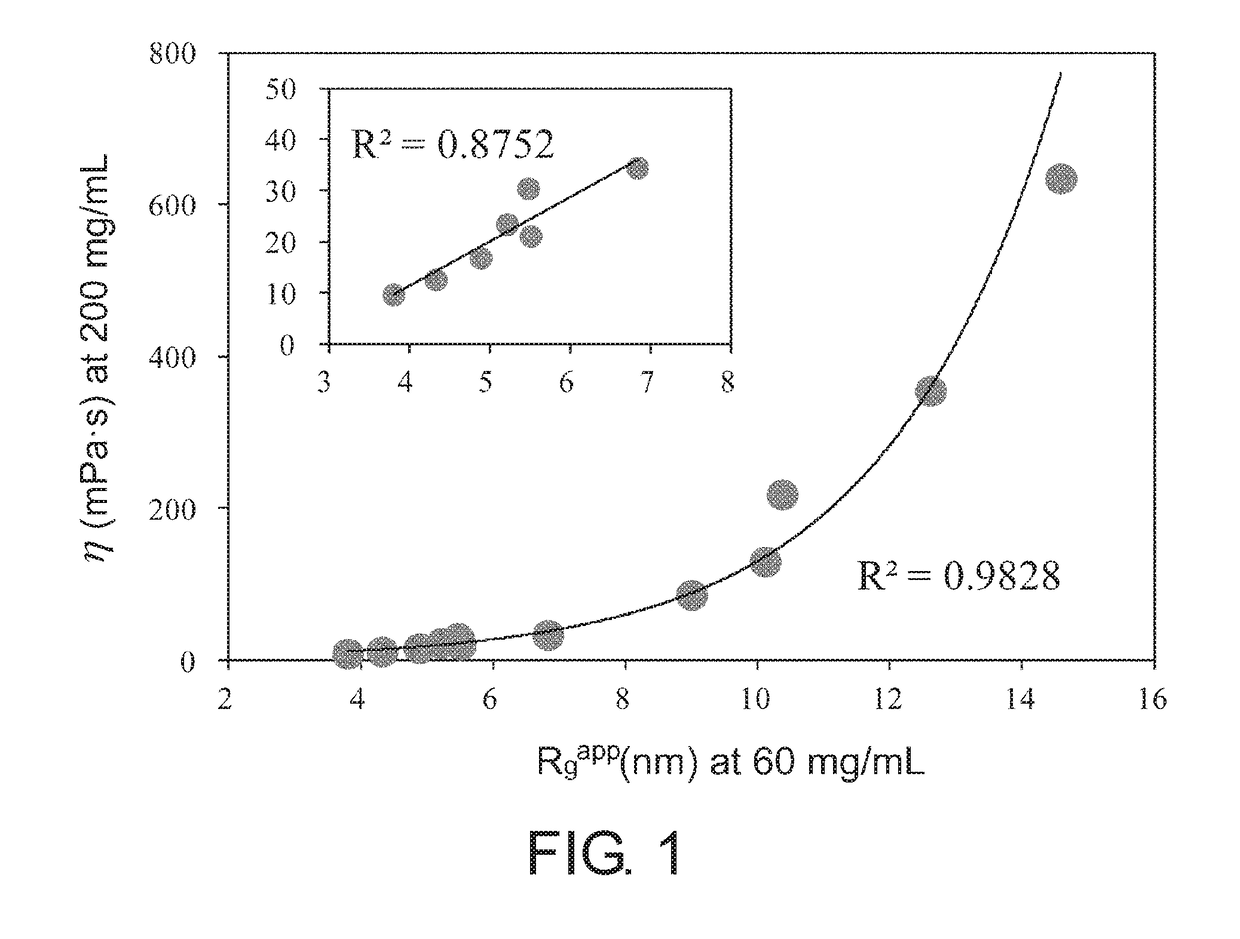
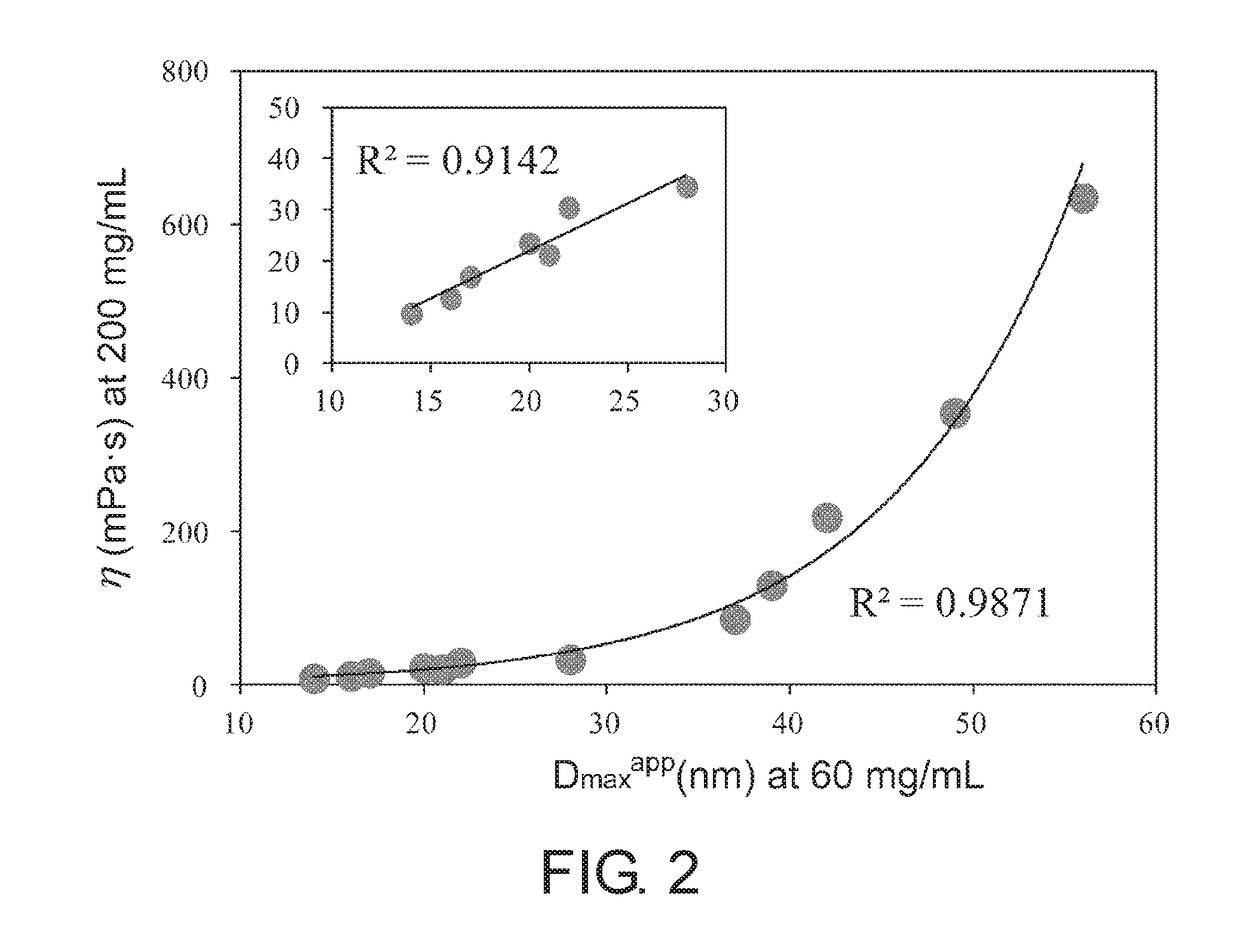
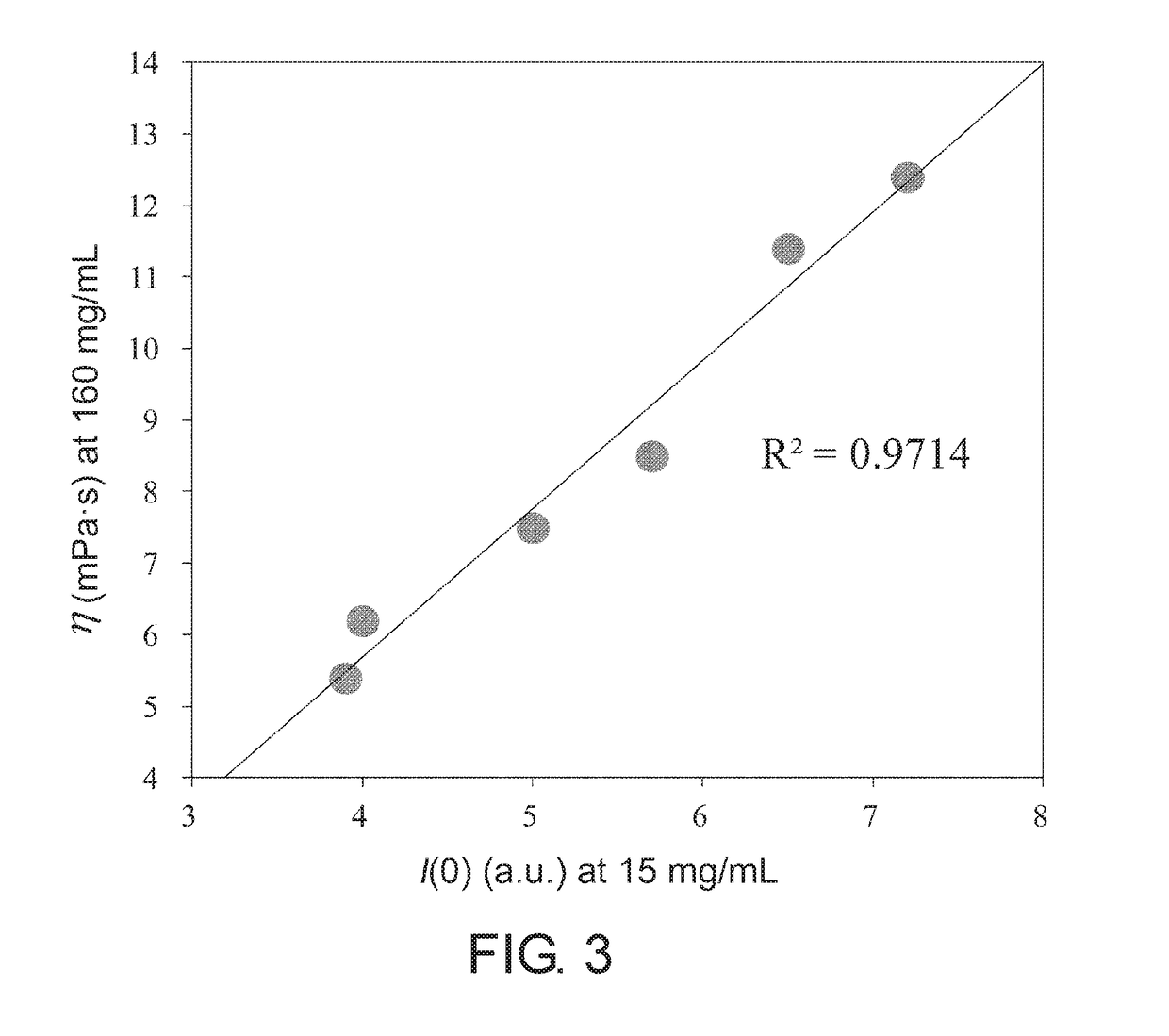
[0098]MAb1, MAb3 (both are the same as those described in Example 1), and MAb4 (humanized anti-IL-6 receptor antibody; generic name: tocilizumab) were used to prepare samples in which the antibody concentration is 15 mg / mL in the formulated solutions of Table 2. Microcapillary cells were filled with approximately 10 μL of the samples; and the particle size parameters, which are the apparent particle radius of gyration Rgapp (nm) and the apparent maximum particle diameter Dmaxapp (nm), as well as the scattering intensity I(0) (a.u.) at zero scattering angle as a value proportional to the apparent molecular weight were calculated using SAXSess mc2 (Anton Paar). The results appear to show that the larger these values are, the higher the associativity of the antibodies. In order to more clearly detect the difference in associativity between the samples, measurements...
example 3
[Example 3] Correlation Between Particle Size Parameters and Viscosity for MAb2 and MAb4
[0102]MAb2 and MAb4 (same as in Example 1 and Example 2, respectively) were used to examine whether differences in viscosity between the antibody prior to amino acid sequence modification (Mab4) and the antibody in which a portion of the amino acid sequence has been substituted with other amino acids (Mab2) can be evaluated using the measurement methods of the present invention. The respective antibodies were used to prepare samples in which the antibody concentration is 15 mg / mL in the formulated solutions of Table 3. Microcapillary cells were filled with approximately 10 μL of the samples, and the particle size parameters, which are scattering intensity I(0) at zero scattering angle, apparent particle radius of gyration Rgapp, and apparent maximum particle diameter Dmaxapp(nm), were calculated using SAXSess mc2 (Anton Paar). The results appear to show that the larger these values are, the highe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com