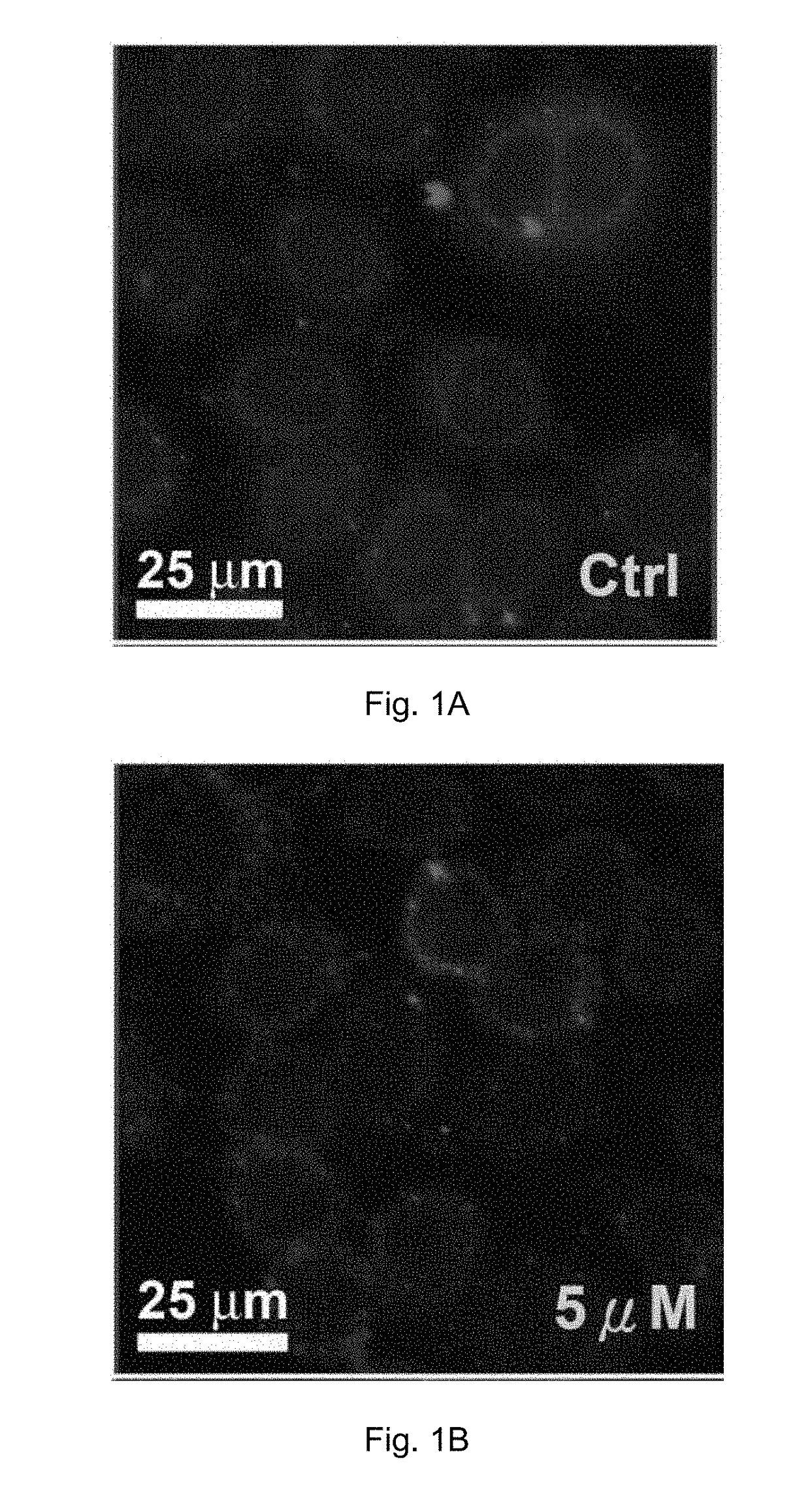
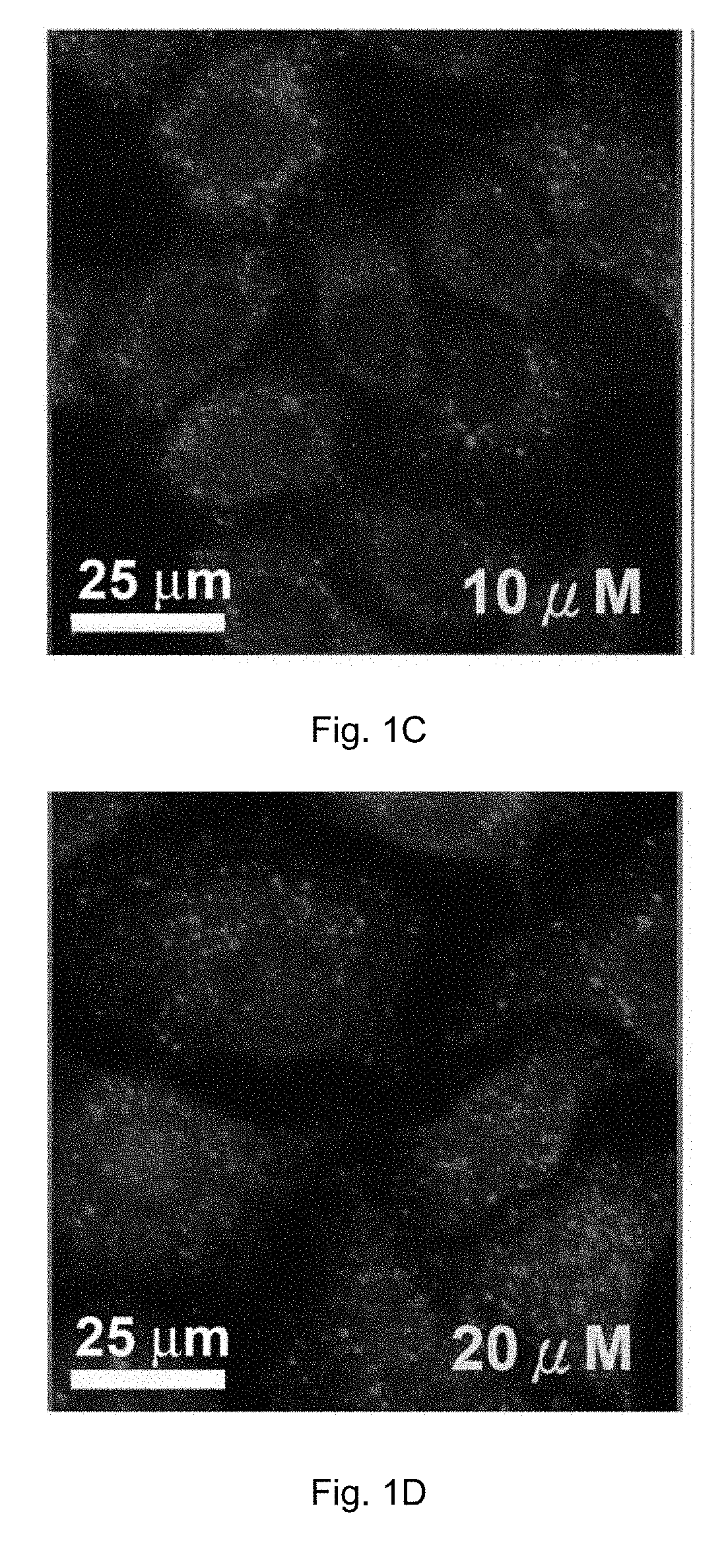
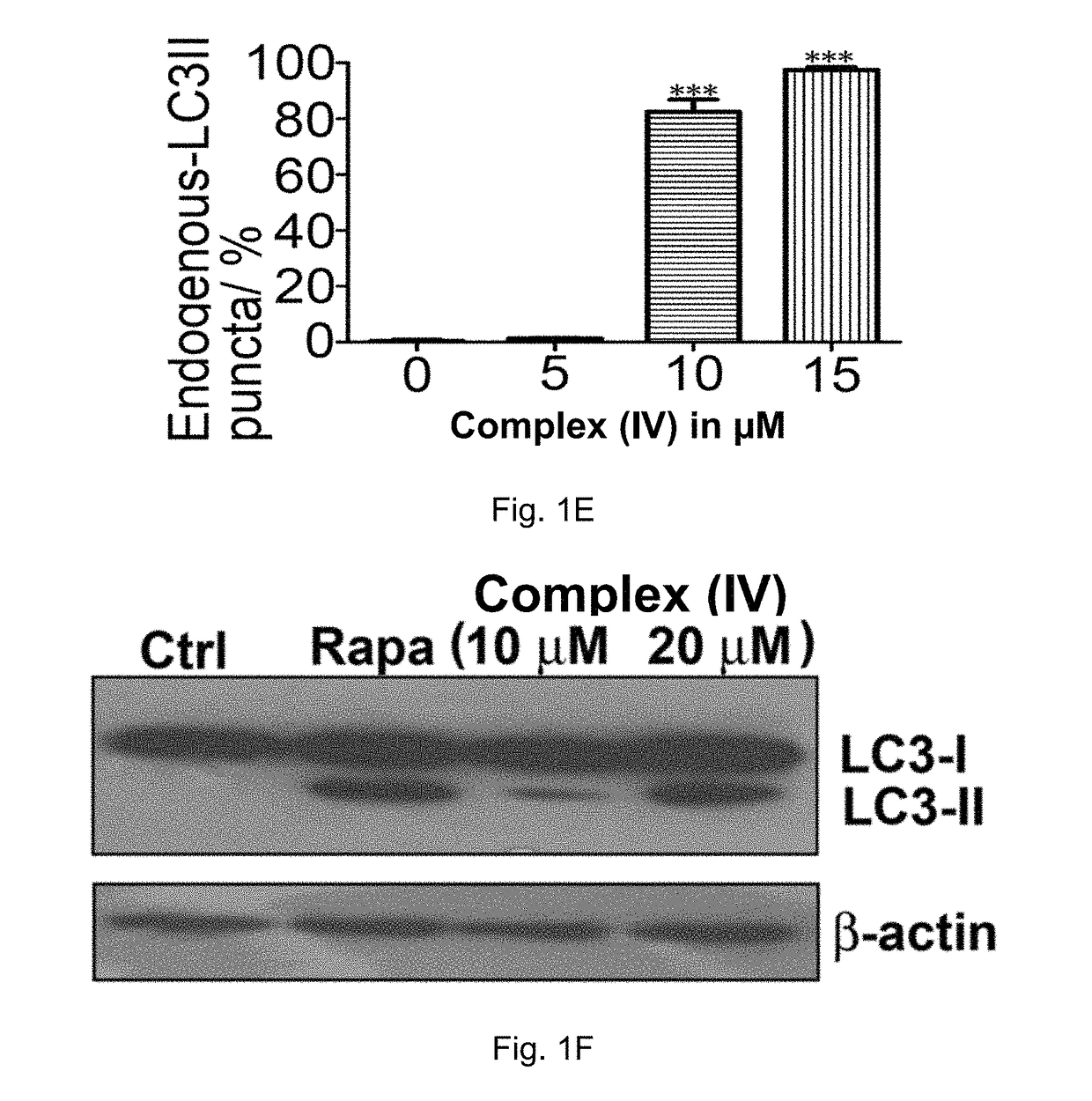
Cobalt-polypyridyl complex for treatment of cancer, a pharmaceutical composition and a kit comprising it
a technology of cobalt polypyridyl complex and cancer, which is applied in the direction of heavy metal compound active ingredients, medical preparations, antineoplastic agents, etc., can solve the problems of dna damage, cancer cell apoptosis, and cancer is still a life-threatening disease affecting an increasing number of people in the world, so as to effectively target cancer and achieve high cytotoxic activity , the effect of reducing the dos
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Cobalt-Polypyridyl Complexes of the Present Invention
[0148]Cobalt-polypyridyl complexes of Formula (V) and (VI) with a counterion were synthesized according to the method below. Other cobalt-polypyridyl complexes were prepared by the modification of the reported procedure.
Preparation of [CoII(N̂N)3](PF6)2 (Cobalt-Polypyridyl Complex of Formula (V) with PF6− as Counterion)
[0149]A mixture of CoC12.6H2O (4 mmol) and 2,2′-bipyridine (13.2 mmol) in methanol (100 mL) was heated to reflux under N2 for 2 h. After cooling down to room temperature, NH4PF6 (20 mmol) was added to the reaction mixture and the reaction mixture was stirred for another 1 h. The precipitate was filtrated and washed with methanol and then diethyl ether.
Preparation of [CoIII(N̂N)3](PF6)3 (Cobalt-Polypyridyl Complex of Formula (VI) with PF6− as Counterion)
[0150]Oxidation of [CoII(N̂N)3](PF6)2 (2 mmol) was carried out by the treatment of NOBF4 (2 mmol) in acetonitrile (40 mL) at room temperature for 1 h. ...
example 2
Cytotoxicity Assays
[0155]All prepared cobalt-polypyridyl complexes were dissolved in DMSO at a final concentration of 50 mmol / L and stored at −20° C. before use. Cytotoxicity was assessed by using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (5.0 mg / ml) assay as previously described (Wong, V. K. et al., Cell Death Dis, 2013, 4, e720). Briefly, 4×103 cells per well were seeded in 96-well plates before drug treatments. After overnight culture, the cells were then exposed to different concentrations of selected compounds (0.039-100 μmol / L) for 72 h. Cells without drug treatment were used as control. Subsequently, MTT (10 μL) solution was added to each well and incubated at 37° C. for 4 h followed by the addition of 100 μL solubilization buffer (10% SDS in 0.01 mol / L HCl) and overnight incubation. A570 nm was then determined in each well on the next day. The percentage of cell viability was calculated using the following formula: Cell viability (%)=Atreated / Aco...
example 3
Collateral Sensitivity in Taxol-Resistant and p53-Deficient Cancer Cells
[0157]All prepared cobalt-polypyridyl complexes were dissolved in DMSO at a final concentration of 50 mmol / L and stored at −20° C. before use. Cytotoxicity was assessed by using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (5.0 mg / ml) assay as previously described (Wong, V. K. et al., Cell Death Dis, 2013, 4, e720). Briefly, 4×103 cells per well were seeded in 96-well plates before drug treatments. After overnight culture, the cells were then exposed to different concentrations of selected compounds (0.039-100 μmol / L) for 72 h. Cells without drug treatment were used as control. Subsequently, MTT (10 μL) solution was added to each well and incubated at 37° C. for 4 h followed by the addition of 100 μL solubilization buffer (10% SDS in 0.01 mol / L HCl) and overnight incubation. A570 nm was then determined in each well on the next day. The percentage of cell viability was calculated using t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com