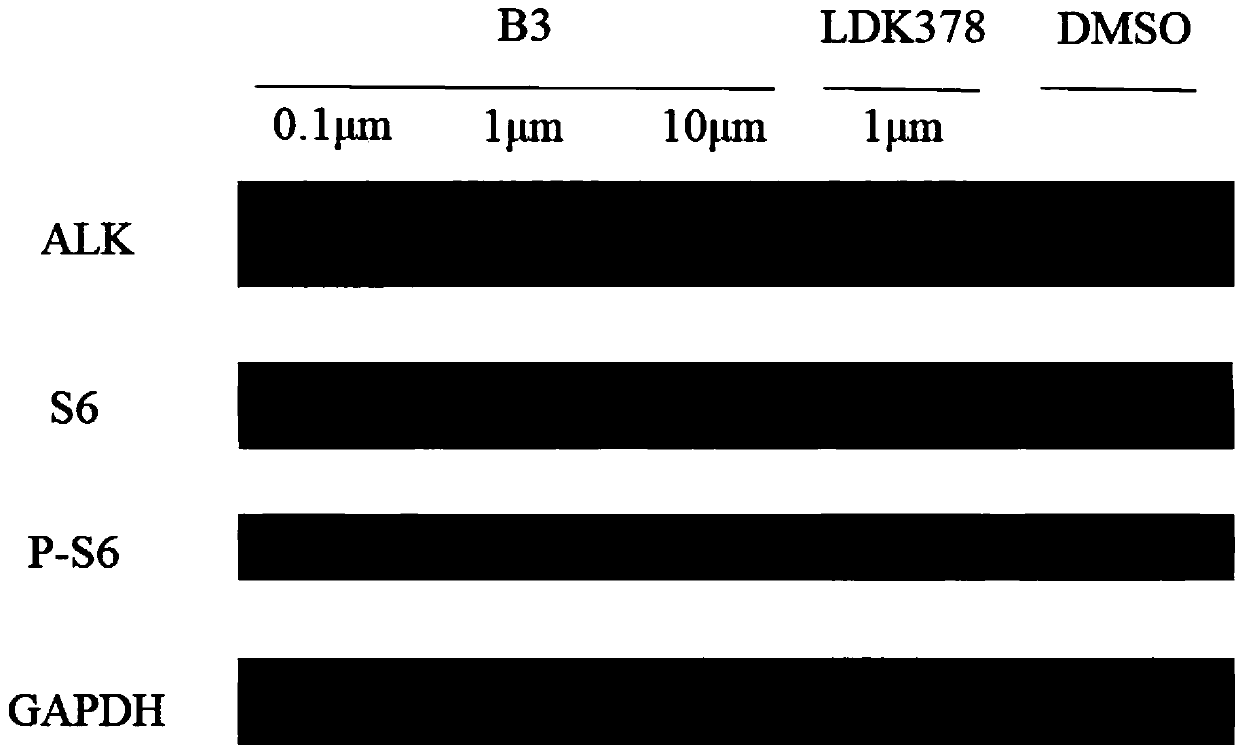
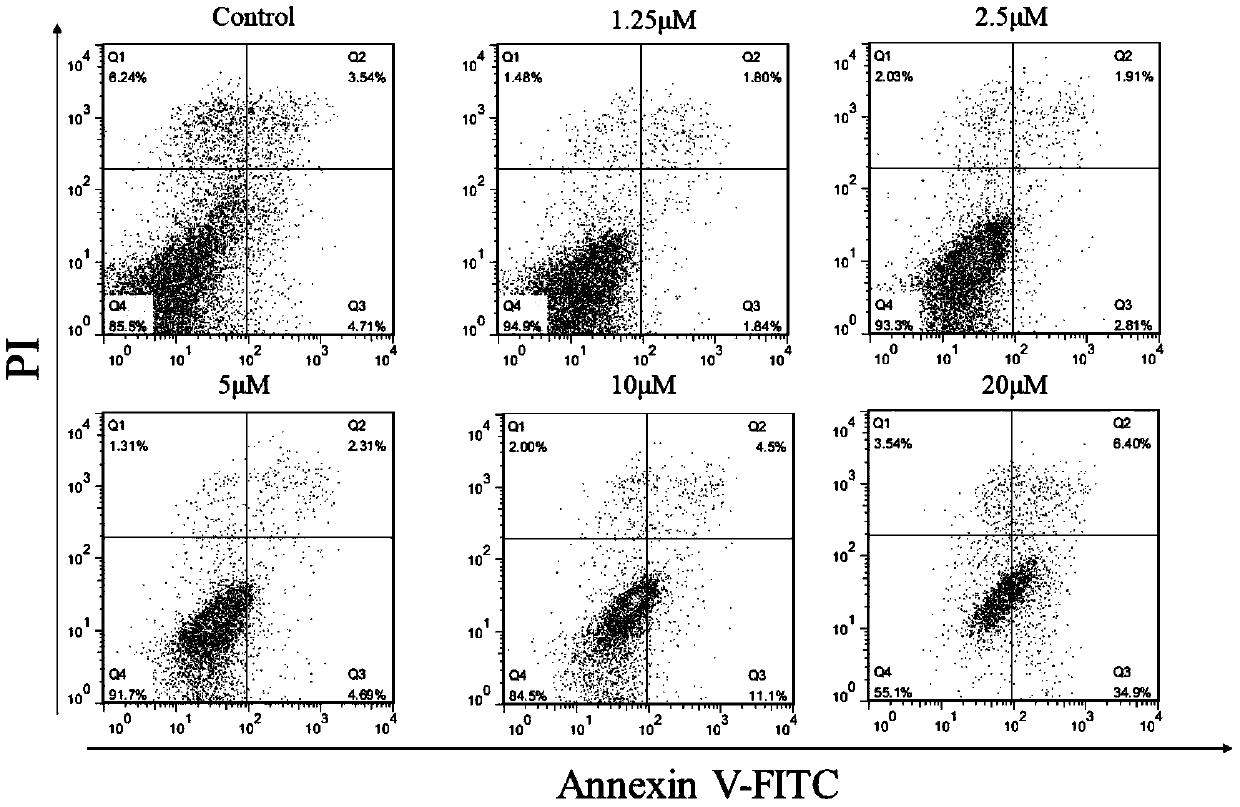
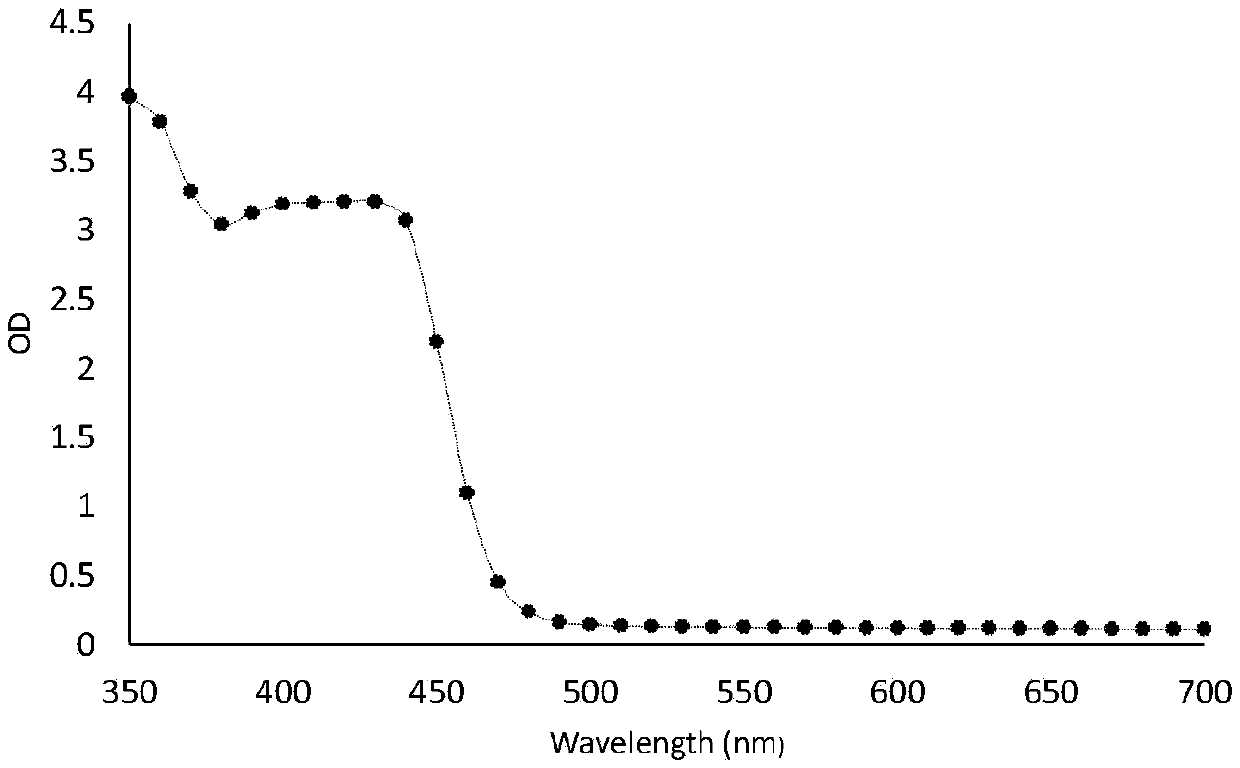
ALK-targeted PROTAC and application thereof
A -CH2-, compound technology, applied in the field of anti-tumor drugs, can solve the problems of decreased kinase activity and anti-tumor activity, and achieve good anti-tumor activity and low toxicity of normal cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] 1. Synthesis of L series molecules
[0045]
[0046] (i) Oxalyl chloride, DMF, rf, 2h; (ii) Triethylamine, DCM, rt, 15h (iii) EDCI, Hobt, DCM, rt, 12h
[0047] Synthesis of intermediate P4
[0048]
[0049] The raw material succinic acid (118 mg, 0.1 mmol) was added to SOCL 2 In, add 2 drops of DMF, heat and reflux for 2h. The reaction solution was dried under reduced pressure at normal temperature to obtain product 1.
[0050] Dissolve the product 1 in anhydrous DCM, add pomalidomide and triethylamine, and react at room temperature for 15h. After the reaction was completed, the reaction was quenched with water, extracted three times with EA, the organic layer was washed twice with saturated brine, dried over anhydrous sodium sulfate, concentrated, and separated by column chromatography to obtain intermediate P4.
[0051] 1H NMR (400MHz, DMSO) δ = 8.47 (d, J = 8.1, 1H), 7.82 (dd, J = 8.4, 7.4, 1H), 7.60 (d, J = 6.9, 1H), 5.14 (dd, J = 12.8,5.4,1H), 2.91(ddd,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com