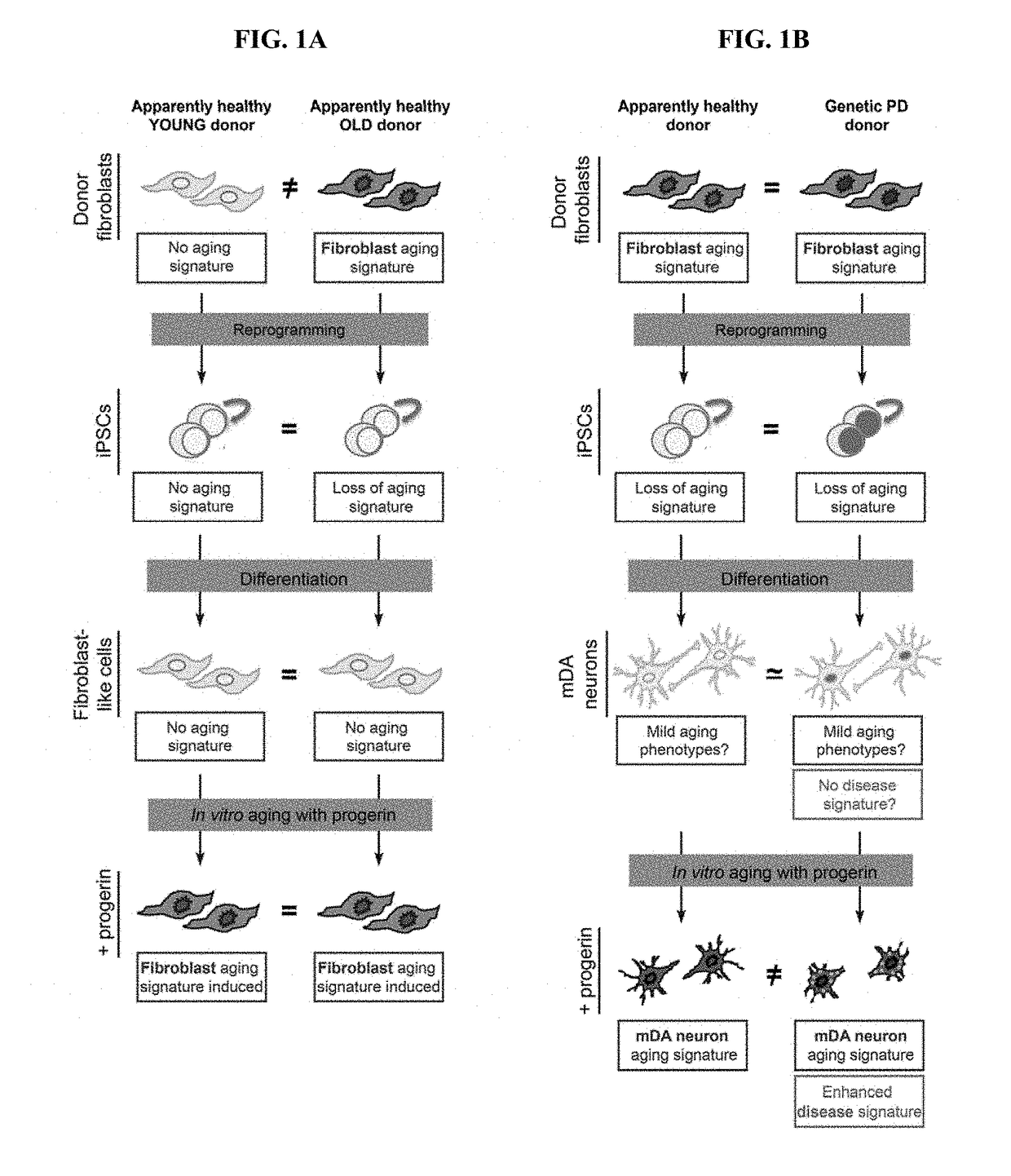
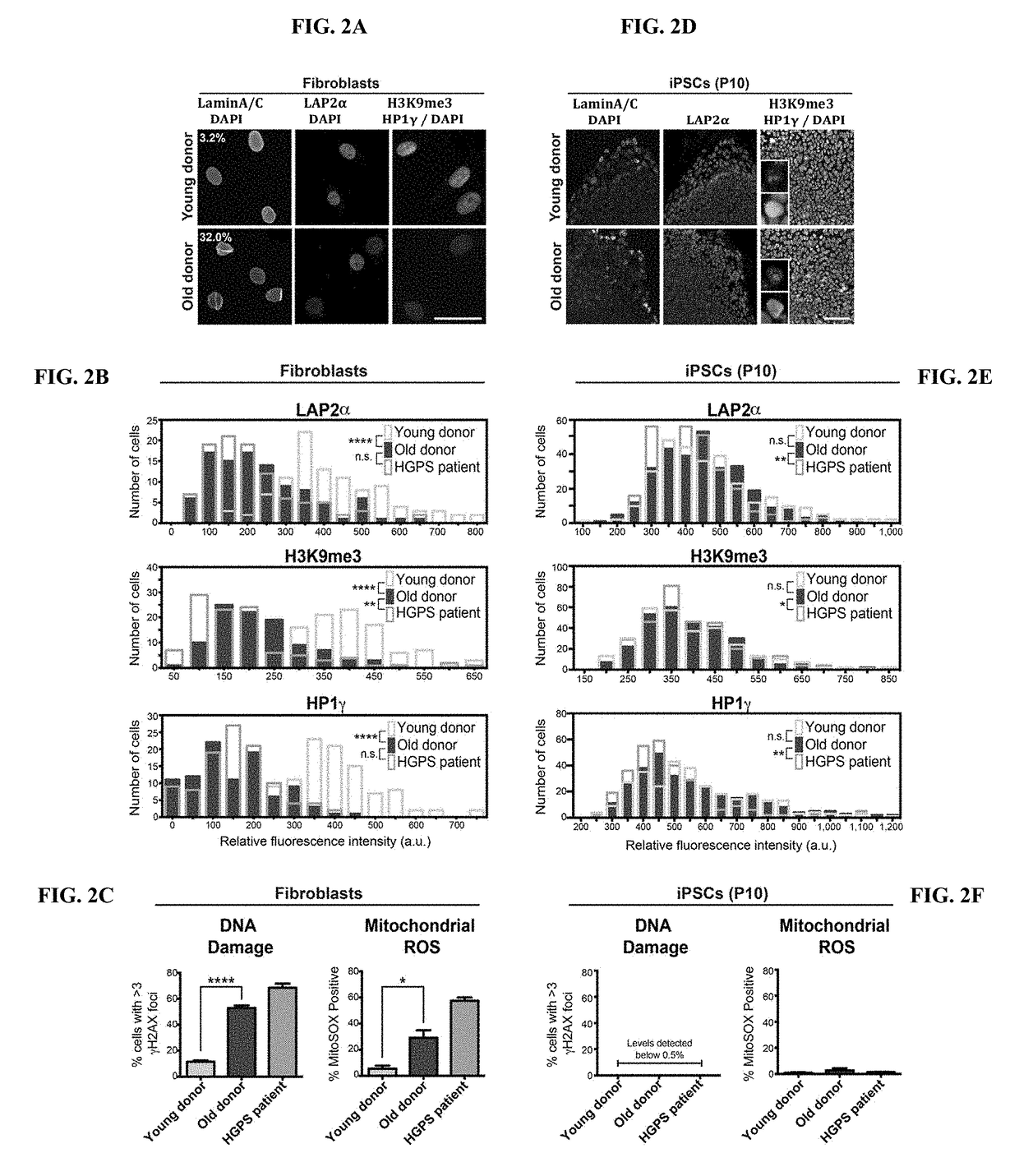
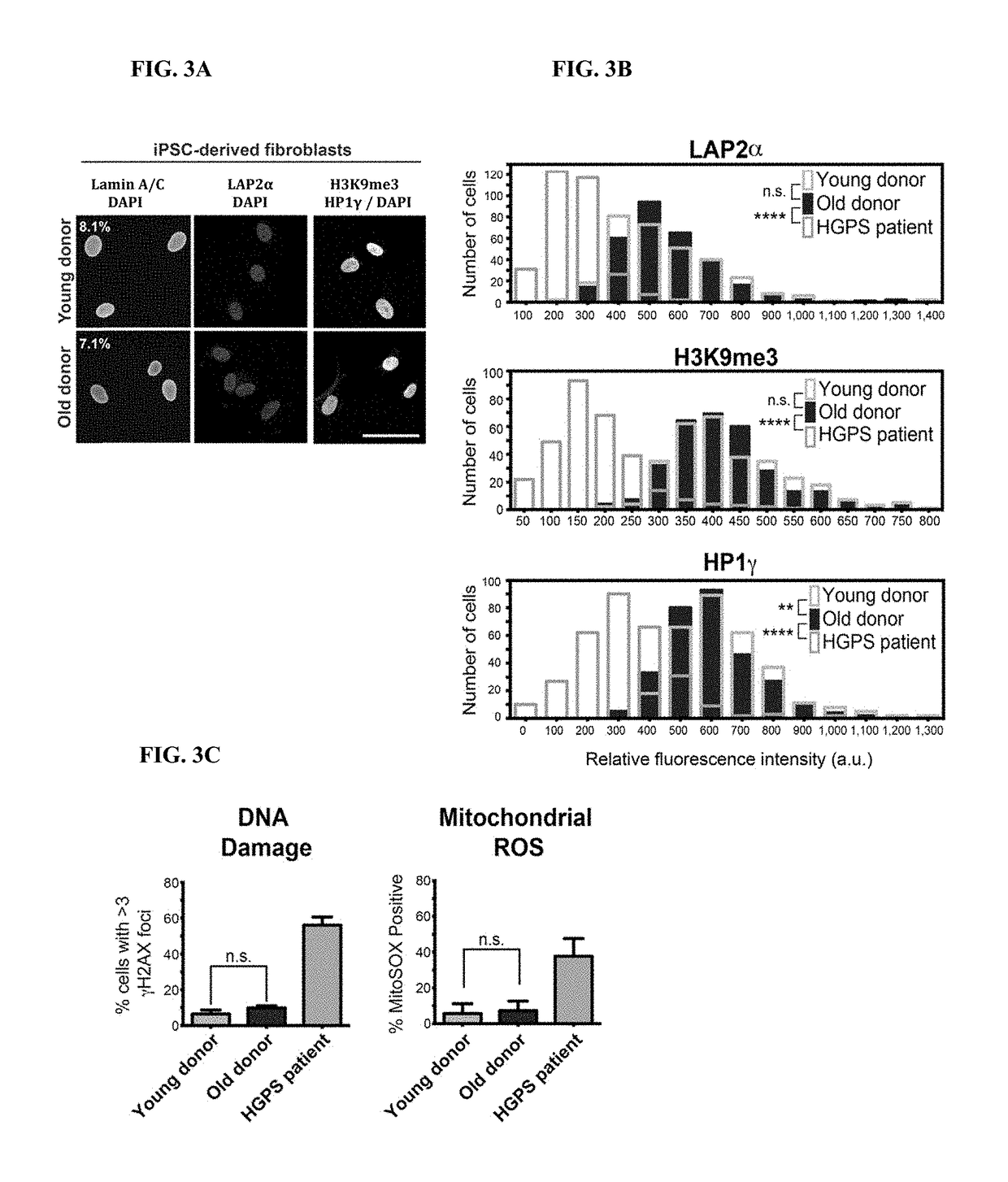
Age-modified cells and methods for making age-modified cells
a technology of age-modified cells and cells, applied in the field of age-modified cells and methods for making age-modified cells, can solve the problems of limited symptom relief, limited treatment options, and increasing burden on society of neurodegenerative disorders such as parkinson's disease or alzheimer's disease, and achieve the effect of accelerating aging and/or maturation of cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Sendai Vector System Reprogramming
[0179]This example describes an integration-free reprogramming technique via a Sendai vector system. Fusaki et al., Proc Japan Acad Ser B:348-362 (2009). This method with such modifications as are described below was used to reprogram somatic cells into iPSC.
[0180]It is believed that this method eliminates concerns about insertional mutagenesis induced by integration of the reprogramming vectors contributing to phenotype. For example, this method has generated an integration-free HGPS iPSC cell line (FIG. 18).
example 2
Progerin Transduction into iPSC-Derived Somatic Cell Culture
[0181]This example illustrates methods for the introduction of a progerin gene into iPSC-derived somatic cultures using a synthetic mRNA approach (Warren et al., Cell Stem Cell 7:618-630 (2010)) or various vectors. Unlike stable transfection techniques, the addition of mRNAs allows for easy manipulation of the duration of gene expression. This advantage is utilized discontinue progerin expression and to monitor the effects following protein turnover (See, FIG. 19). This protocol can be used to introduce any other progerin-like protein into a cell, whether iPSC, primary stem cell, embryonic stem cell or primary or cultured somatic cell not derived from an iPSC.
[0182]The following is a protocol for progerin overexpression using modified-RNA in differentiating or differentiated iPSC-derived cells:[0183]1. Prepare progerin-modified-RNA (methods of synthesis are described in Example 9) and deliver to iPSC-derived somatic cells u...
example 3
Directed Differentiation of Neuronal Cell Types
[0191]This example describes one method of directed differentiation techniques to generate specific neural cell types. Nearly pure populations of CNS lineages, such as midbrain dopamine (mDA) neurons, are used in the methods described herein. The protocol of Kriks et al, Nature 2011, infra, can be used (among other methods).
[0192]Briefly, a modified version of the dual-SMAD inhibition protocol was used to direct cells towards floor plate-based mDA neurons as described previously (Kriks et al., Nature 480:547-551 (2011) and schematized in FIG. 8. iPSC-derived mDA neurons were replated on day 30 of differentiation at 260,000 cells per cm2 on dishes pre-coated with polyornithine (PO; 15 μg / ml) / Laminin (1 μg / ml) / Fibronectin (2 μg / ml) in Neurobasal / B27 / L-glutamine-containing medium (NB / B27; Life Technologies) supplemented with 10 μM Y-27632 (until day 32) and with BDNF (brain-derived neurotrophic factor, 20 ng / ml; R&D), ascorbic acid (AA; 0....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com