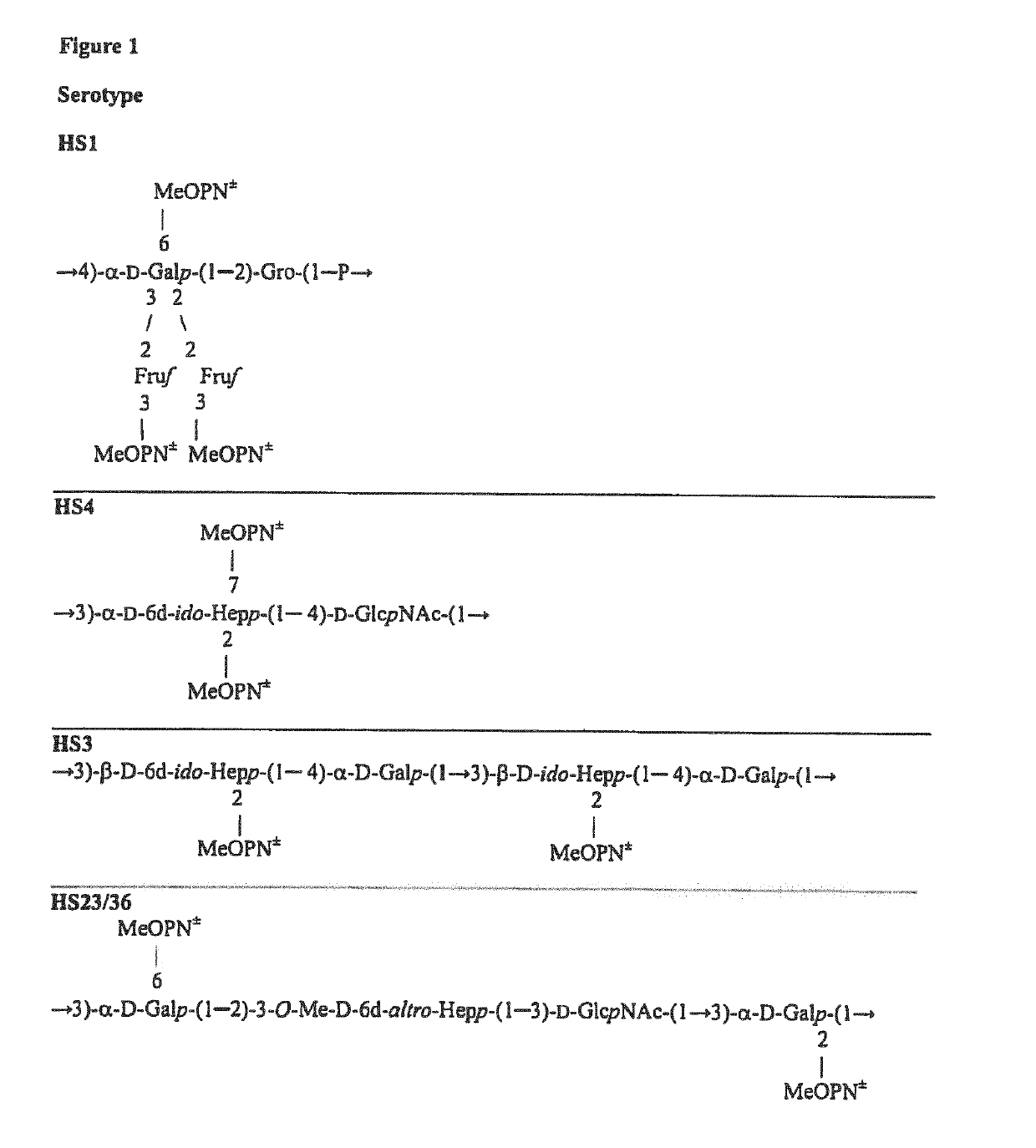
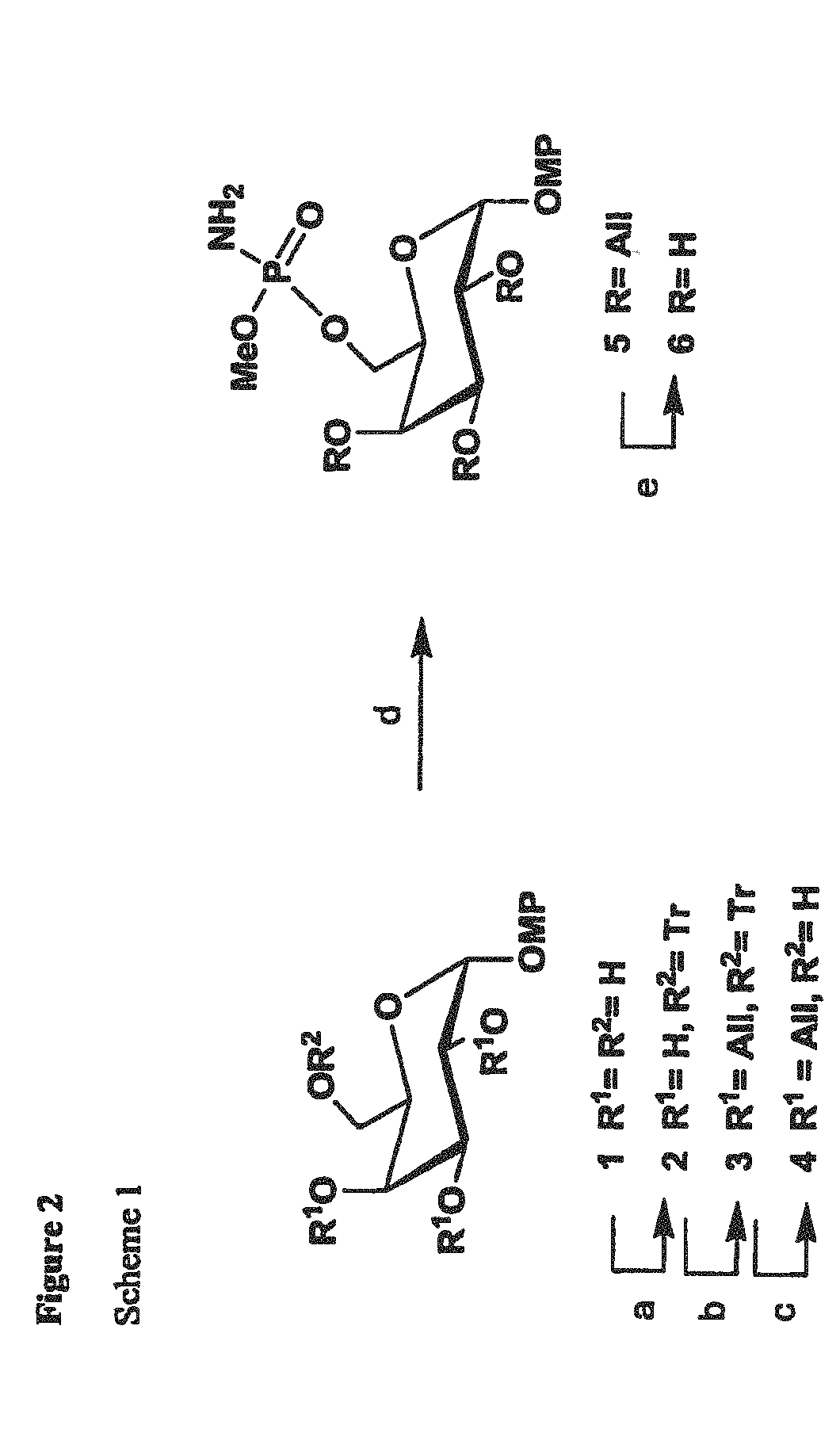
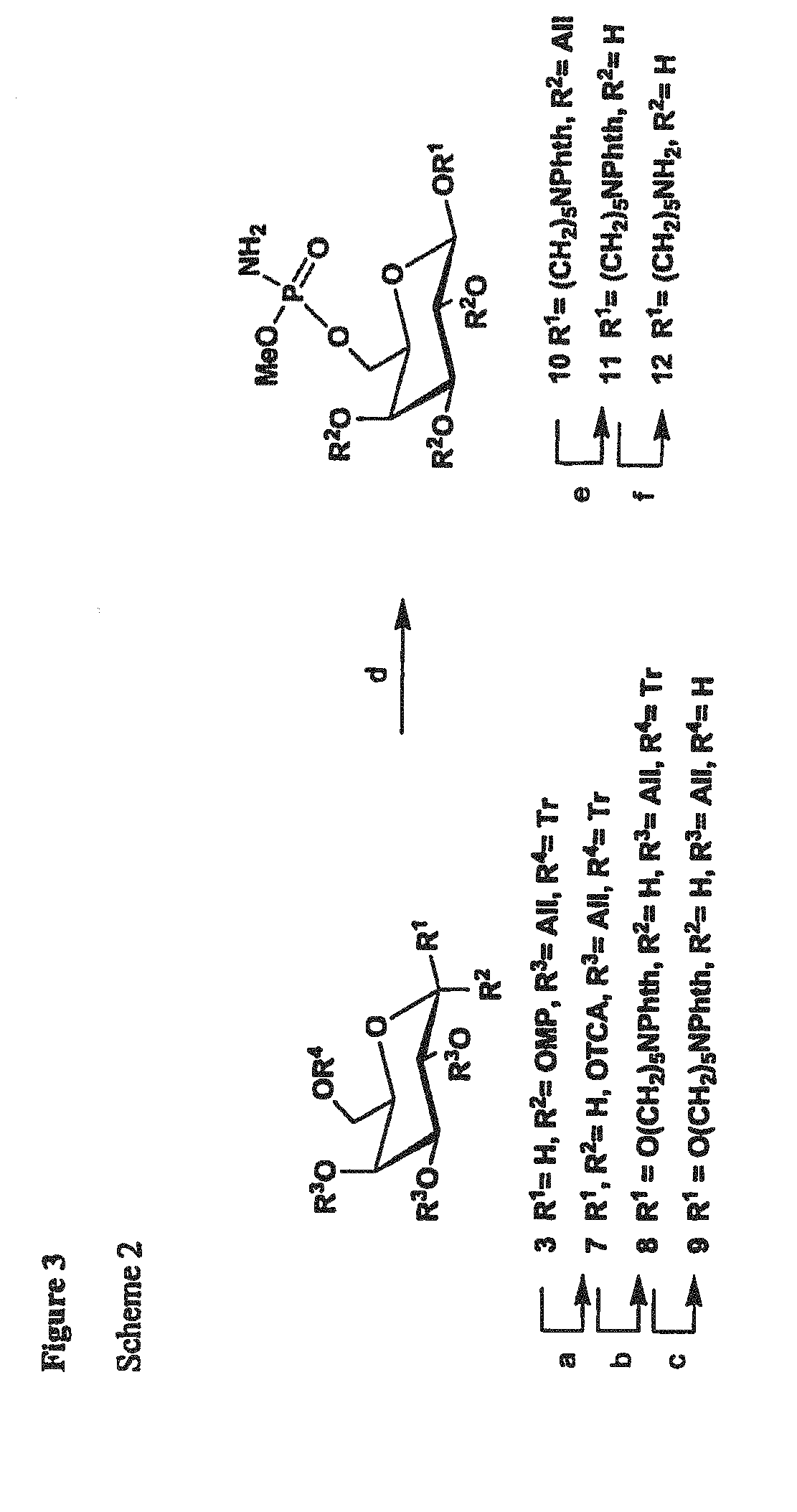
Synthetic Antigen Constructs Against Campylobacter Jejuni
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of p-methoxyphenyl and aminopentyl glycosides of the MeOPN→6-Gal Construct: MeOPN→6-α-D-Galp-(1→OMP and MeOPN→6-β-D-Galp-(1→O(CH2)5NH2
[0142]Previously, using conventional methods and mass spectrometry, we detected a non-stoichiometric MeOPN unit at the 2 position of galactose (MeOPN-2-Gal) in C. jejuni 81-176 CPS, with a 31P resonance similar to that depicted in FIG. 20A (peak Y) (Kanipes M I, et al. (2006.) J. Bacteriol. 188:3273-3279.) We confirmed this MeOPN-2-Gal linkage by NMR (FIG. 21A) through the detection of a cross-peak between the 31P resonance Y (δP 14.45) of MeOPN and H-2 (δH 4.52) of the galactose unit in a 1H-31P correlation experiment. In some 81-176 CPS preparations, albeit of lower intensity, the 31P NMR spectrum displayed an additional resonance at δP 14.15 (designated peak Z) (FIG. 20B). A similar peak was also observed in another 81-176 CPS preparation (a mutant in gene CJJ81176_1420) that exhibited a cross-peak between the phosphorous of MeOPN and H-...
example 2
Synthesis of MeOPN→2-β-D-Galp-(1→OMP
Summary Synthesis of MeOPN→2-β-D-Galp-(1→OMP (FIG. 5, Scheme 3)
[0178]The synthesis of MeOPN→2-β-D-Galp-(1→OMP is depicted in FIG. 5, scheme 3. The synthesis of galactoside (product 7) began with a known compound, 4-methoxyphenyl 3,4-O-isopropylidene-6-O-trityl-β-D-galactopyranoside (product 1), which was prepared from D-galactose following published procedures (Scheme 1.) (Comfort D A, et al., Biochem 2007; 46:3319-3330.) To distinguish the C-2 position, O-allylation was performed generating product 2 in excellent yield. Since MeOPN can be removed by acidic media, suitable protecting groups needed to be installed. Thus, O-isopropylidene and O-trityl groups were removed giving product 3, which was then per-benzoylated affording product 4. Nast, the allyl group was removed yielding a free 2-OH for modification. The introduction of MeOPN group to product 5 followed a strategy developed in our lab, involving first a phosphorylation with commercially a...
example 3
Immunodetection of MeOPN→6-α-D-Galp-(1→OMP and MeOPN→6-β-D-Galp-(1→O(CH2)5NH2 by C. jejuni CPS Conjugate Antisera
[0188]The synthetic p-methoxyphenyl and aminopentyl glycosides of the MeOPN→6-Gal construct, compounds MeOPN→6-α-D-Gal-(1→OMP and MeOPN→6-β-D-Galp-(1→O(CH2)5NH2, synthesized as described in the above examples, were tested per reactivity with antisera previously raised against C. jejuni CPS conjugates of serotypes HS1, HS3, HS4 and HS23 / 36. Notably, of the listed serotypes, only HS23 / 36 expresses MeOPN-6-Gal.
Materials and Methods
[0189]The synthetic construct MeOPN-6-Gal was adjusted to 1 mg / ml and 2 μl was spotted onto nitrocellulose membranes and allowed to dry. The individual spots were immunodetected with four different polyclonal antisera made against different conventional conjugate vaccines in which different C. jejuni polysaccharide capsules were conjugated to CRM197: (1) rabbit serum against an HS23 / 36 conjugate (final dilution 1:1000 in 20 mM Tris, pH 7.4, 0.425 M...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com