Competitive inhibitors of invariant chain expression and/or ectopic clip binding
a competitive inhibitor and invariant chain technology, applied in the field of immunology, can solve the problems of unappreciated full impact of these molecules on immune signaling and activation, etc., and achieve the effect of easy synthesis and effective targeting methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Identification of CLIP Inhibitors
[0392]Peptide that are able to displace CLIP were identified using computer based analysis. Thus, examples of “ideal” MHC class II binding peptides were generated according to the invention. Analysis of the binding interaction between MHC class II and CLIP was used to identify other molecules that may bind to MHC class II and displace CLIP. The methods described herein are based on feeding peptide sequences into software that predicts MHC Class II binding regions in an antigen sequence using quantitative matrices as described in Singh, H. and Raghava, G. P. S. (2001), “ProPred: prediction of HLA-DR binding sites.”Bioinformatics, 17(12), 1236-37.
[0393]Because MHC class II HLA-DR can bind to peptides of varying length an analysis of MHC class II HLA-DR-CLIP binding was performed. Since the alpha chain of HLA-DR is much less polymorphic than the beta chain of HLA-DR, the HLA-DR beta chain (hence, HLA-DRB) was studied in more detail. Peptide binding data...
example 2
B-Cell Apoptosis after Coxsackievirus Infection
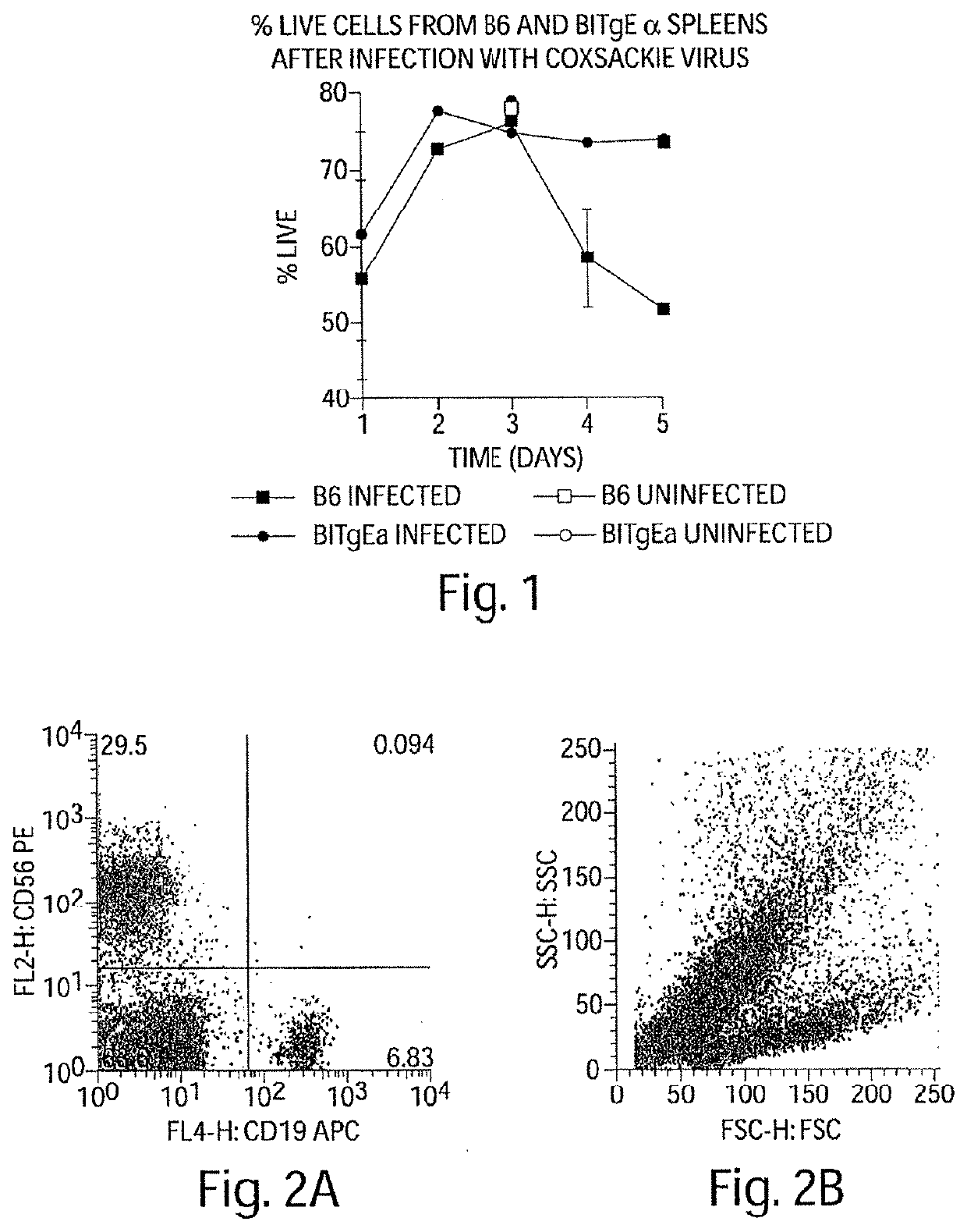
[0410]During the course of Coxsackievirus infection, animals that recover from the virus without subsequent autoimmune sequalae have high percentages of splenic B cell apoptosis during the infection in vivo (FIG. 1). Those animals susceptible to Coxsackievirus-mediated autoimmune disease have non-specifically activated B cells that do not undergo apoptosis, at least not during acute infection, nor during the time period prior to autoimmune symptoms indicating that a common feature in the development of autoimmune disease is failure of non-specifically activated B cells to die.
example 3
Activated B Cells in HIV Disease Mediate NK Cell Activation
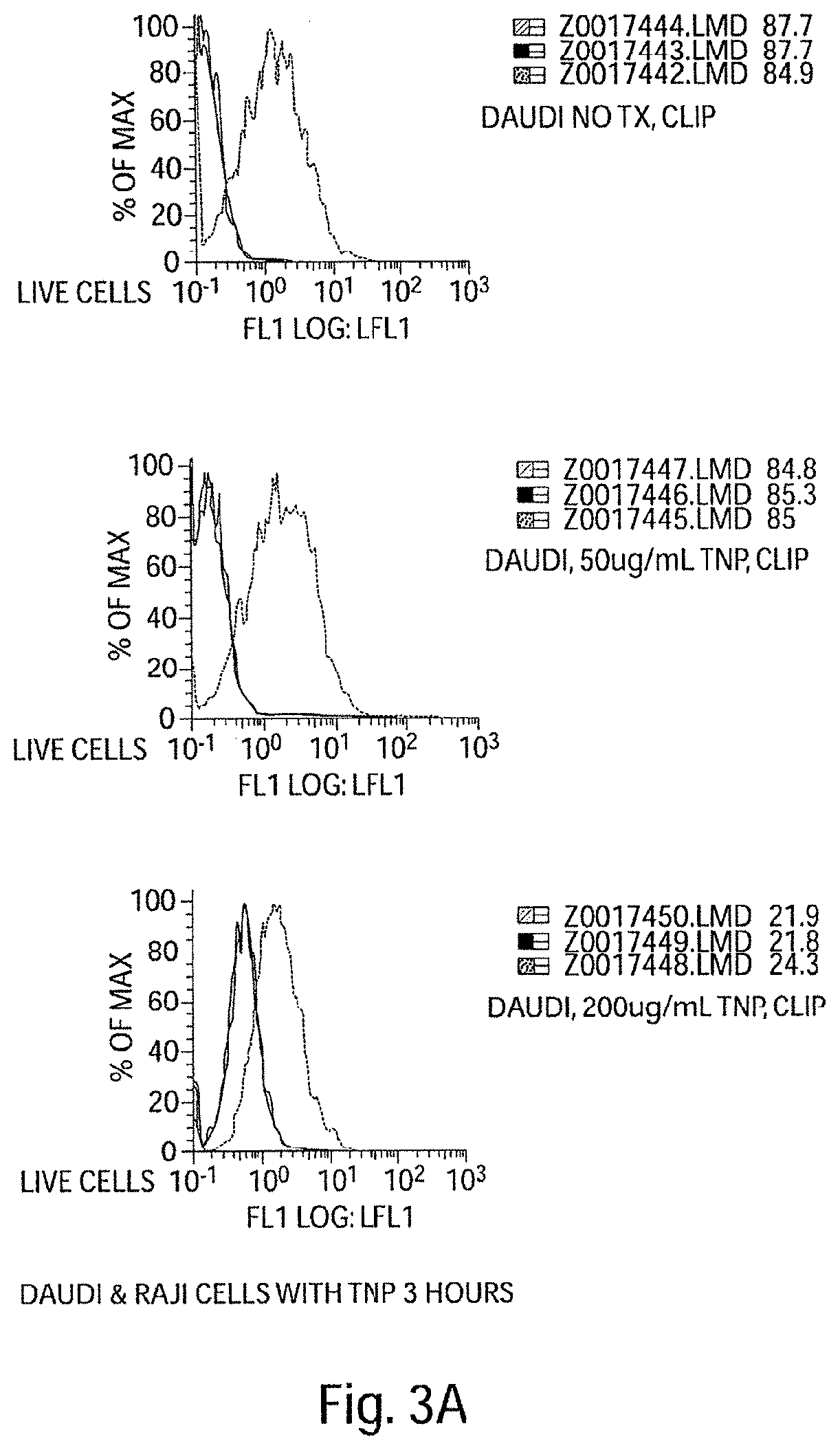
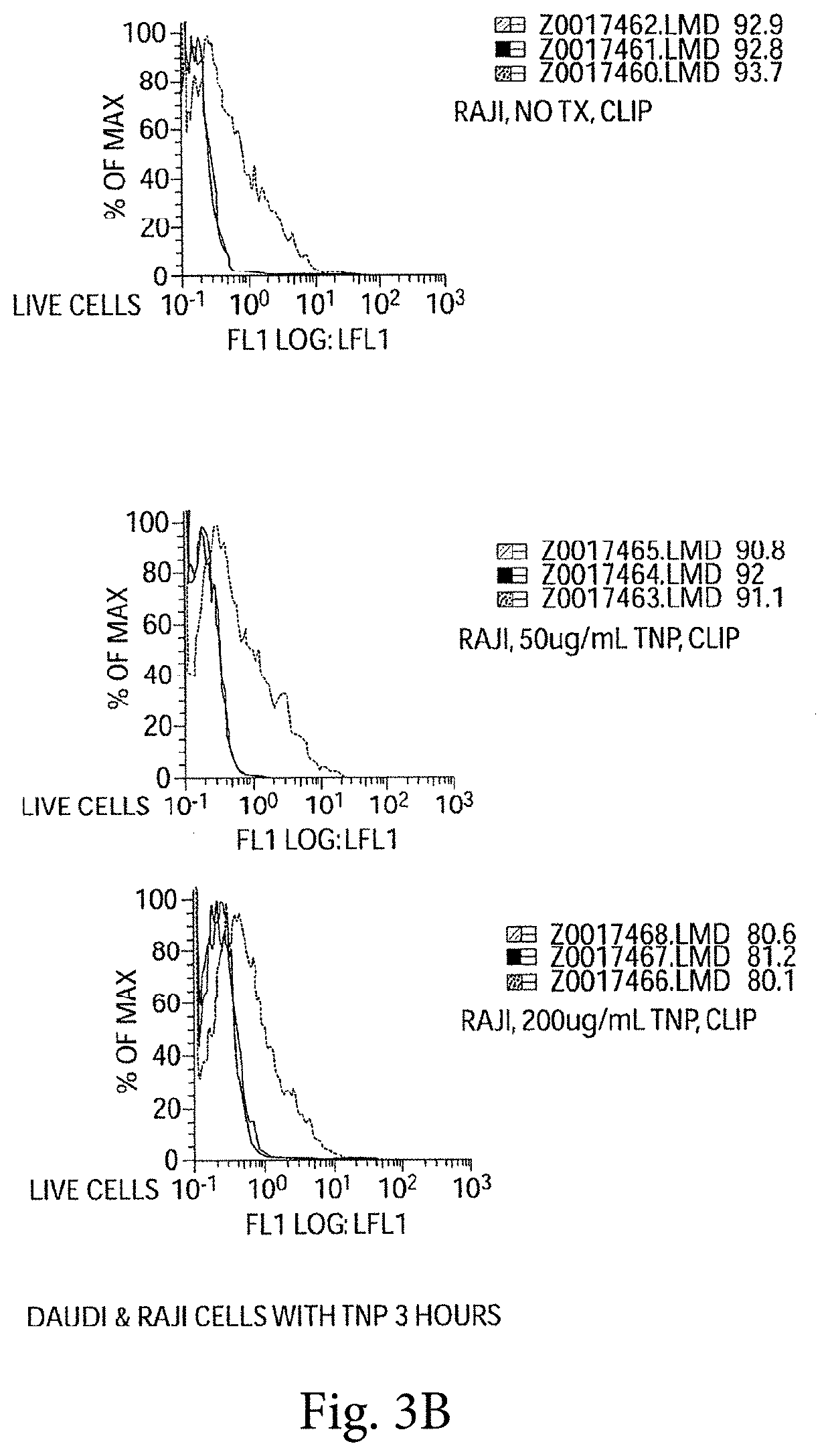
[0411]We experimentally induced polyclonal activation of peripheral blood human B cells in an antigen-independent fashion using a combination of CD40 engagement (CD40Ligand bearing fibroblasts) and culture in recombinant IL-4. We isolated the activated B cells and return them to co-culture with autologous peripheral blood mononuclear cells (PBMCs). After five days of co-culture, we observed a striking increase in the percentage of activated NK cells in the PBMC culture (NK cells accounting for up to 25-50%, FIG. 2a, of the surviving PBMCs), and a dramatic apoptotic loss of the activated B cells (FIG. 2b). These data indicate that antigen—independent activated B cells in HIV disease initially activate NK cells.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com