Cannabis Sativa Derived Formulation for Transmucosal and Transdermal Delivery
a technology of cannabis sativa and transdermal delivery, which is applied in the direction of capsule delivery, plant/algae/fungi/lichens ingredients, oil/fat/waxes non-active ingredients, etc., can solve the problems of affecting the bioavailability of cbd, not considered the most acceptable route of administration, and unhealthy choice of cdb administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Serum Levels of Nano-Encapsulated Vs Non-Encapsulated CBD
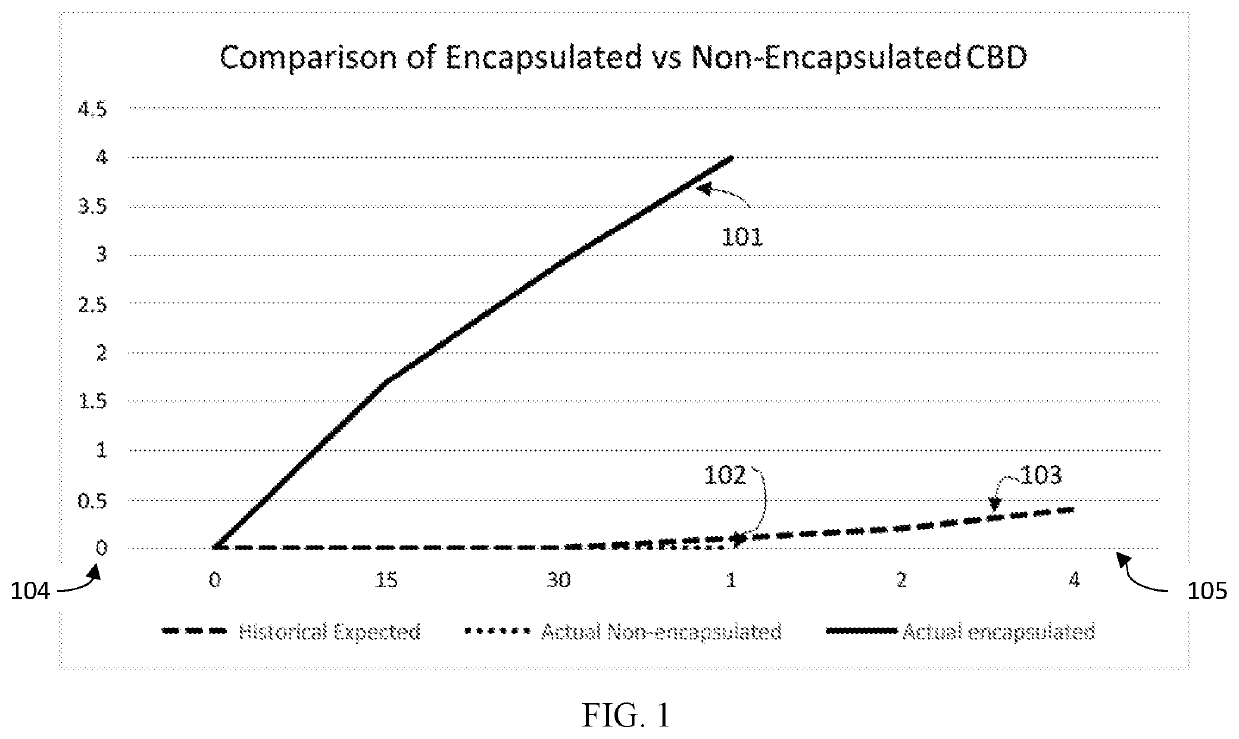
[0080]For transmucosal administration, CBD was encapsulated in a nanosized phospholipid vesicle for the nanoencapsulated preparation, and for comparison purposes, CBD was combined with an olive oil carrier for a non-encapsulated control preparation. The nanoencapsulation vesicles were prepared with a shelf-stable ethanolic stock comprised of water, phospholipids and ethanol, or a similar solvent. Lecithin was used as a source of the phospholipids. The crystalline CBD for use as a control may be dispersed in olive oil, coconut oil or other orally acceptable oil.
[0081]Cannabinoids have a greatly increased bioavailability when encapsulated in nanosized phospholipid vesicles as compared to non-encapsulated cannabinoids. FIG. 1 shows a comparison of serum levels achieved for oromucosal administration of cannabidiol encapsulated in nanosized phospholipid vesicles and a control of cannabidiol mixed in olive oil.
[0082]In the FIG. 1 gr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com