Novel aerosol formulations of ondansetron and uses thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
on of Spray Dried Ondansetron Fine Powder
[0150]Powdered ondansetron was prepared by spray drying with SPRAY DRYER SD-MICRO™ (manufactured by GEA Process Engineering, Inc., Columbia, Md., USA). The experiments were done at GEA Process Engineering, Inc., Columbia, Md., USA.
TABLE 1Parameters of Spray Drying to Prepare Ondansetron Fine PowderInletOutletSprayNozzleConc.N2Temp.Temp.RateDiameterRun(wt %)(kg / hr)(° C.)(° C.)(g / min)(mm)12.2730170855.80.522.2730170853.40.532.27301951005.00.5
example 2
Size Distribution of Spray Dried Ondansetron Fine Powder
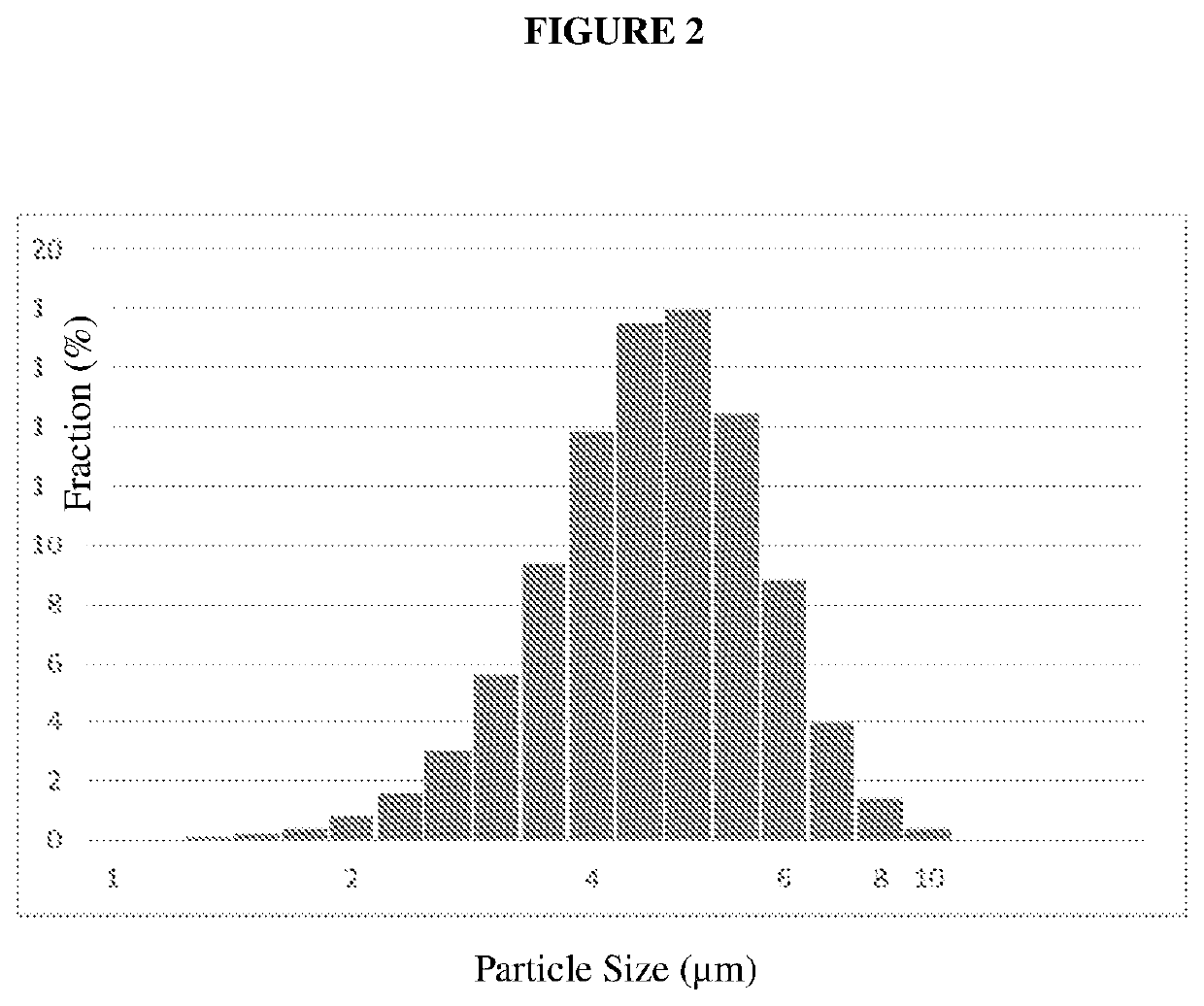
[0151]The Particle Size Distribution of the Ondansetron Fine Powder, prepared by Spray Drying using the above parameters was measured by Malvern Mastersizer (Malvern Instruments, UK) at GEA Process Engineering, Inc., Columbia, Md., USA.
[0152]The typical Particle Size Distribution:
[0153]Mean Size: 4.80 μm
[0154]Std. Dev.: 1.59 μm
[0155]D10: 3.23 μm
[0156]D50: 5.01 μm
[0157]D90: 7.22 μm
[0158]Cumulative % on <10 μm: 99.5%
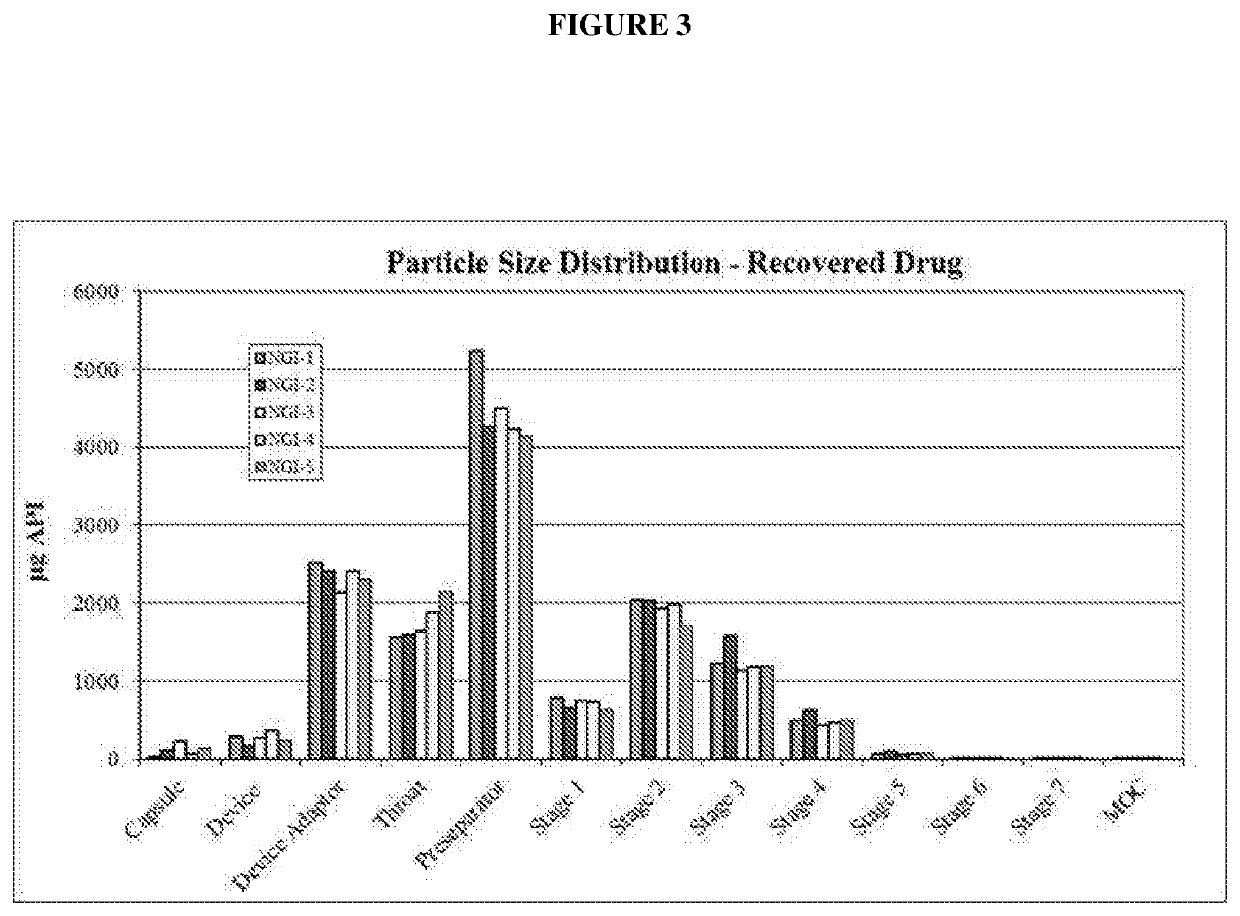
[0159]FIG. 2 shows the typical Particle Size Distribution of the Spray Dried Ondansetron.
example 3
icle Fraction by Laser Diffraction
[0160]The Fine Particle Fraction (FPF) of the dry powder ondansetron aerosol formulations was measured by Laser Diffraction at Drug Dynamics Institute, College of Pharmacy, The University of Texas at Austin, Austin, Tex., USA.
[0161]The dry powder formulation measured by NGI included:
[0162]Spray Dried Ondansetron (SDO): 14 mg;
[0163]Excipient: None.
[0164]A Malvern Spraytec equipped with an inhalation cell and induction port was used for measuring the aerosol emitted from a HandiHaler® operated at 60 LPM. Neat SDO was filled into size 3 hypromellose capsules and inserted into the HandiHaler®. Measurements were carried out over a 4 second duration at 10 measurements / second. The Refractive Index used for SDO was 1.68 with an imaginary index of 0.01. Particles were assumed to be spherical.
[0165]The results showed that the FPF (defined as % <5.41 μm) of the dry powder ondansetron formulation was 15.6%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com