Nucleic acid molecules encoding an engineered antigen receptor and an inhibitory nucleic acid molecule and methods of use thereof
a technology antigen receptors, which is applied in the field of nucleic acid molecules encoding an engineered antigen receptor and an inhibitory nucleic acid molecule, can solve the problems of limited and limited approach to adopting immunotherapy, and achieve the effect of enhancing the allogenicity of enhancing the genetically modified human t cell
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
NK Cell Killing of B2M Knockout Primary Human T Cells
1. Methods and Materials
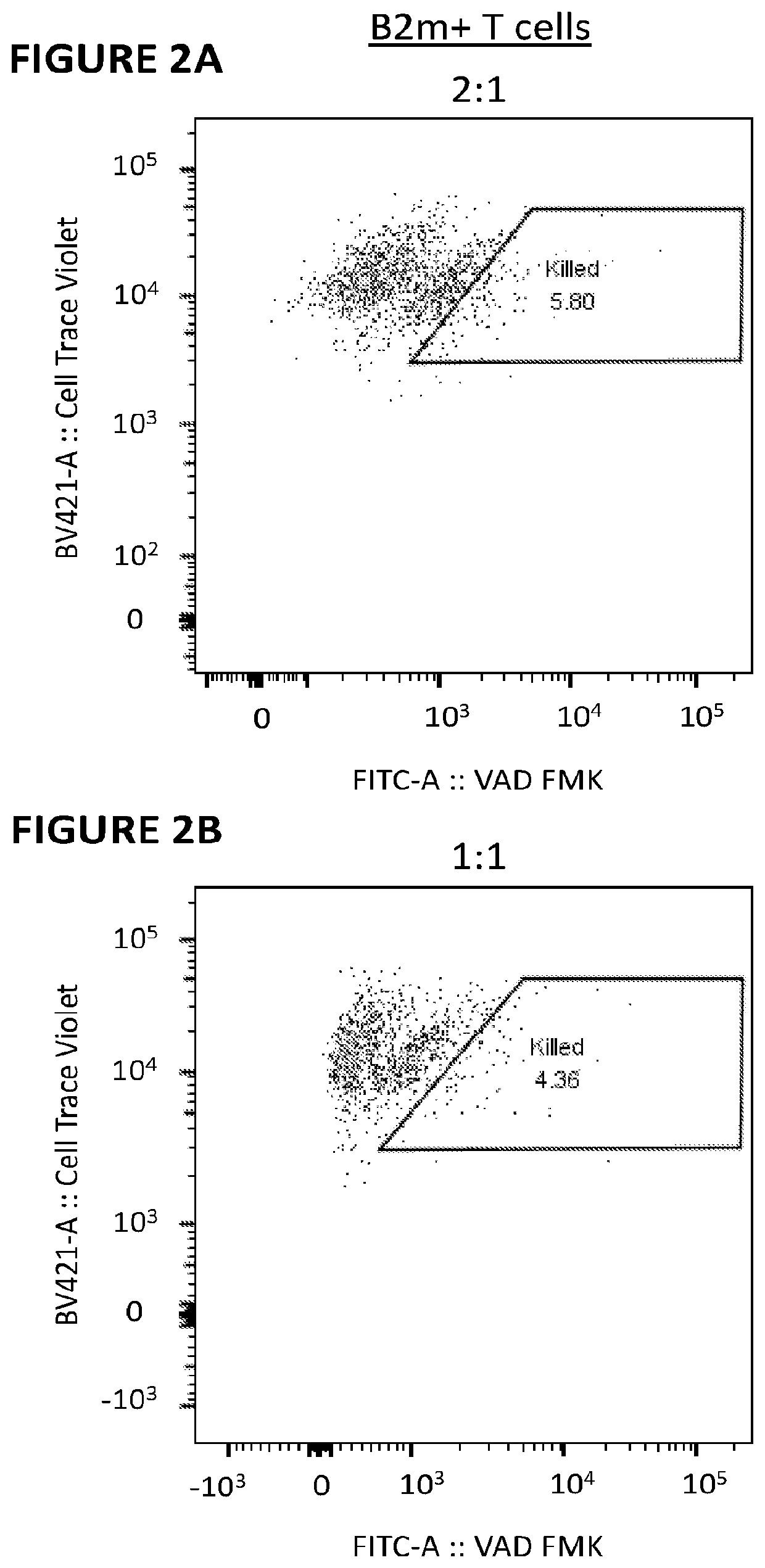
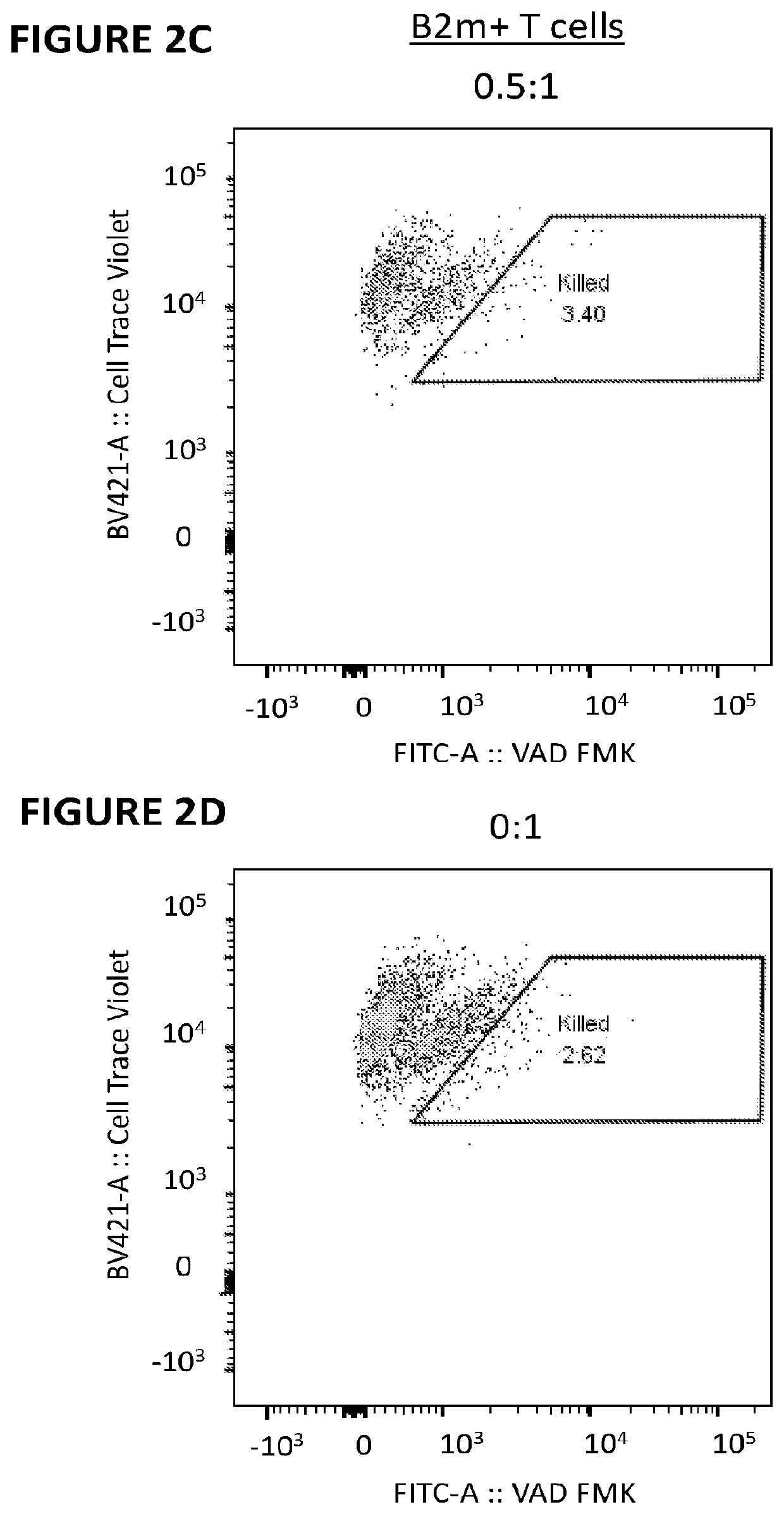
[0300]Primary human T cells were stimulated for 3 days using ImmunoCult anti-CD2 / CD3 / CD28 (StemCell Technologies) in the presence of IL-2 (Gibco) in XVIVO-15 medium (Lonza) supplemented with 5% fetal bovine serum. RNA encoding B2M13-14x479 nuclease was introduced into the T cells using the 4D Nucleofector (Lonza). Cells were cultured in the presence of IL-2 for 6 days before magnetic depletion of remaining B2M+ cells using biotinylated anti-human B2M (BioLegend) and a Biotin Selection Kit (StemCell Technologies). NK cells were isolated from PBMC samples of the same donor using a CD56 positive selection kit (StemCell Technologies). Daudi cells were purchased from ATCC. Daudi cells are naturally B2M− and are reported to be highly sensitive to NK cytolysis. All target cells were labeled with 1 uM CellTrace Violet (LifeTechnologies) to distinguish them from effectors in mixed cultures. Isolated NK cells were mi...
example 2
Characterization of Candidate shRNAs Against B2M and Effect of B2M Knockdown on NK Cell Killing of Primary Human T Cells
1. Materials and Methods
[0303]Five Mission-shRNA lentiviral transfer plasmids encoding different B2M targeting sequences were purchased from Sigma-Aldrich. Second-generation lentiviral vectors were produced in-house using Lenti-X 293T cells (ClonTech) and a triple transfection method (Lipofectamine 2000—Thermo-Fisher). T cells were prepared for lentiviral transduction by stimulating for 3 days with ImmunoCult anti-CD2 / CD3 / CD28 as in Example 1. Transduction was carried out in the presence of 5 uM polybrene (Sigma-Aldritch) and transduced cells were selected with puromycin (InVivoGen) beginning at 48 h post-transduction and concluding 72 h following drug addition. Selected cells were expanded for 5 days in IL-2 supplemented medium before a flow cytometric analysis of B2M surface expression to determine the extent of knockdown. Cultures receiving B2M shRNAs were used ...
example 3
Production and Characterization of CAR T Cells Utilizing shRNA to Reduce Cell Surface Expression of B2M
1. Materials and Methods
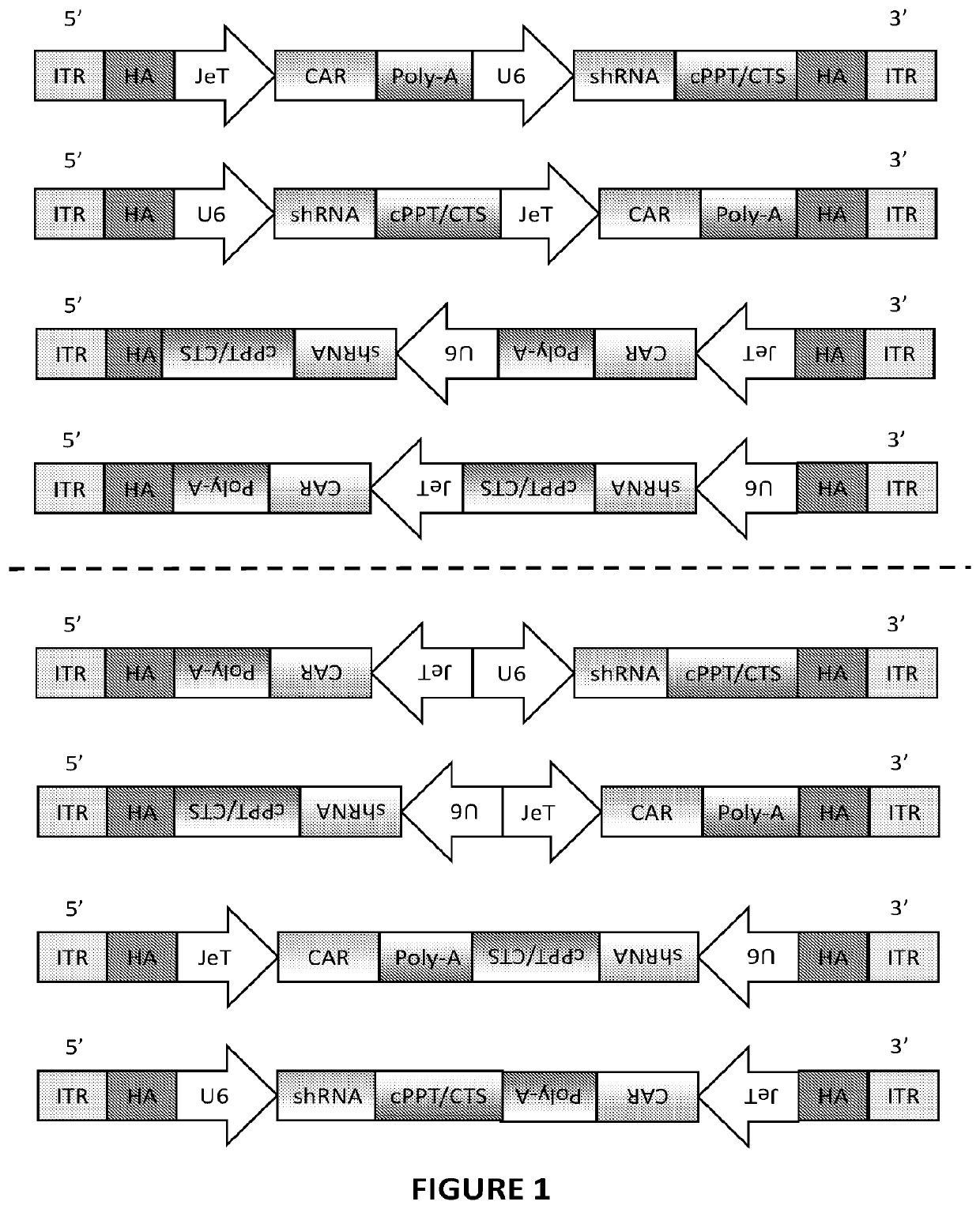
[0307]A number of constructs were prepared comprising an anti-CD19 CAR coding sequence and an shRNA against B2M. These are illustrated in FIG. 7A-7F and are provided in SEQ ID NOs: 18-23. CAR constructs 7007 and 7217 (SEQ ID NOs: 18 and 19) comprise the CAR coding sequence and the shRNA472 sequence in the same 5′ to 3′ orientation. CAR constructs 7008 and 7218 (SEQ ID NOs: 20 and 21) comprise the CAR coding sequence in the 3′ to 5′ orientation, and shRNA472 sequence in the 5′ to 3′ orientation (i.e., tail-to-tail). CAR constructs 7009 and 7219 (SEQ ID NOs: 22 and 23) comprise both the CAR coding sequence and the shRNA472 sequence in the 3′ to 5′ orientation. The 5′ and 3′ homology arms flanking the CAR coding sequence and the shRNA472 sequence have homology to regions upstream and downstream of the TRC 1-2 recognition sequence in the TRAC locus.
[0308]CAR T c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com