Method of predicting and determining therapeutic effect on rheumatoid arthritis due to biological formulation
a biological formulation and rheumatoid arthritis technology, applied in the field of predicting and determining the therapeutic effect of a biological formulation on a rheumatoid arthritis patient, can solve the problems of limited use cases of biological formulations, high cost, inaccurate estimation, etc., and achieve the effect of improving the level of symptoms, accurate estimation, and high precision
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0168]Hereinafter, the present invention is disclosed in detail while using Examples to facilitate the understanding thereof. However, the Examples are not provided to limit the present invention, but only for illustrative purposes. Thus, it is not intended that specifically described embodiments and examples be construed as limitations on the scope of the invention except as set forth in the appended claims.
1. Patient and Experimental Method
(Patient)
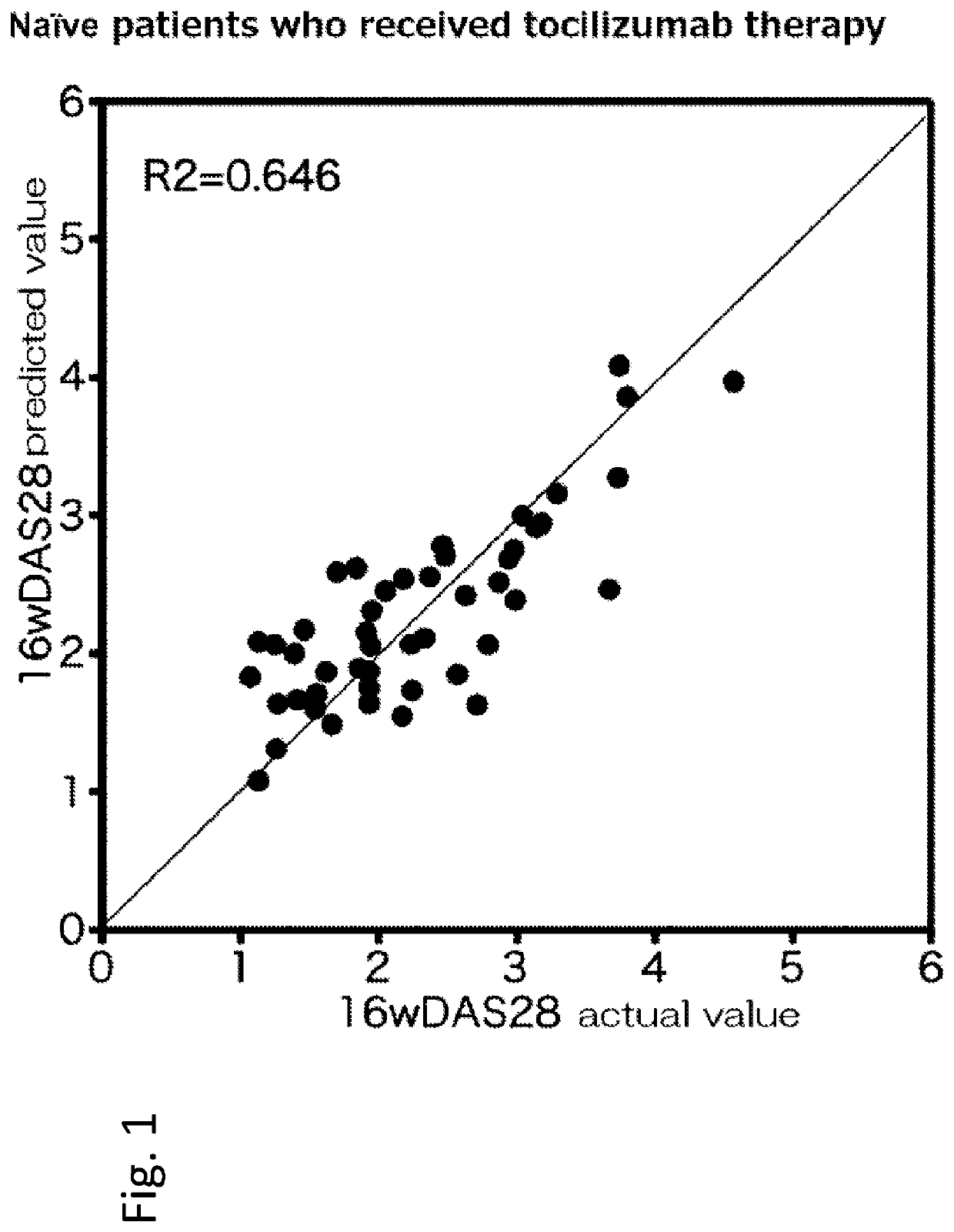
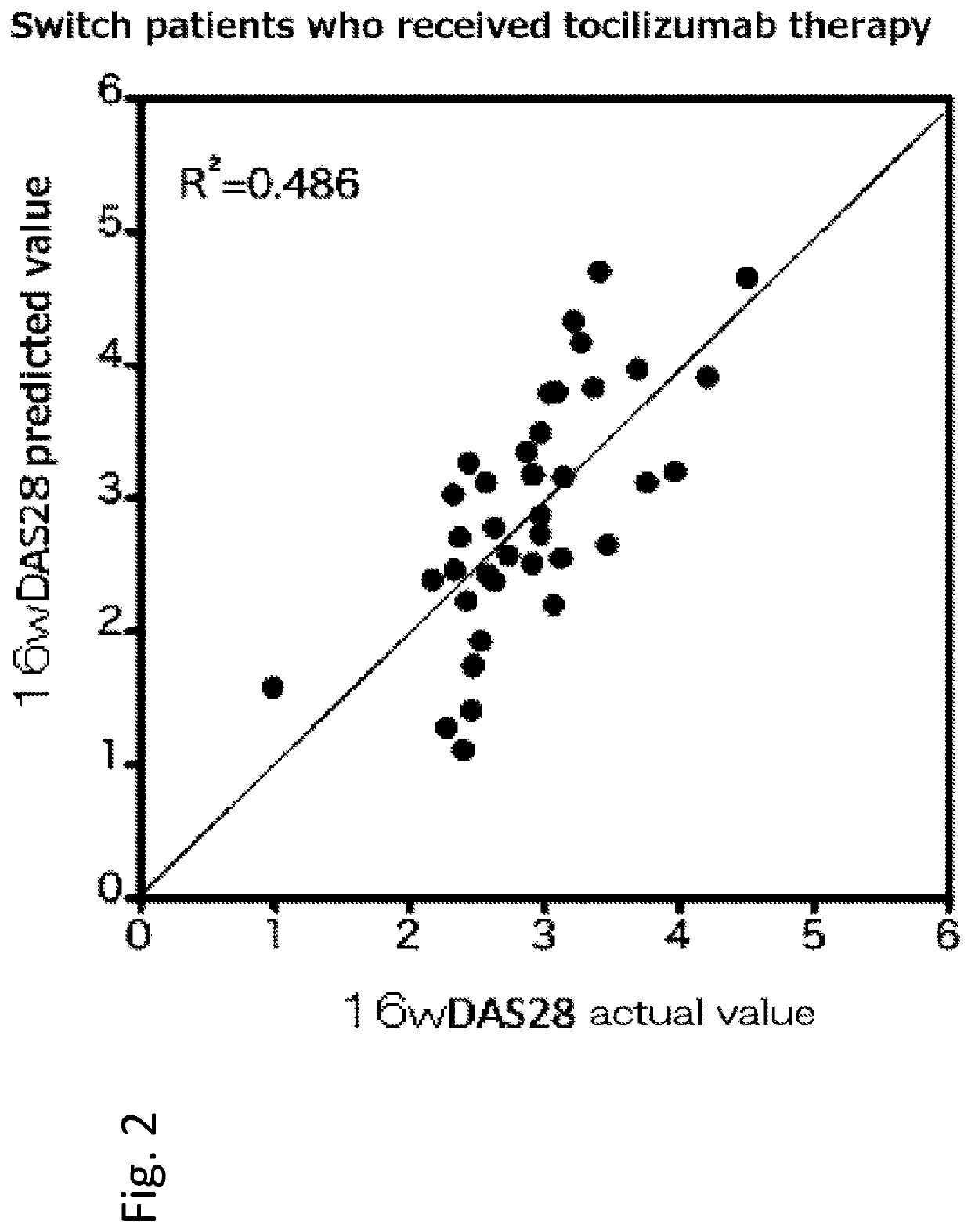
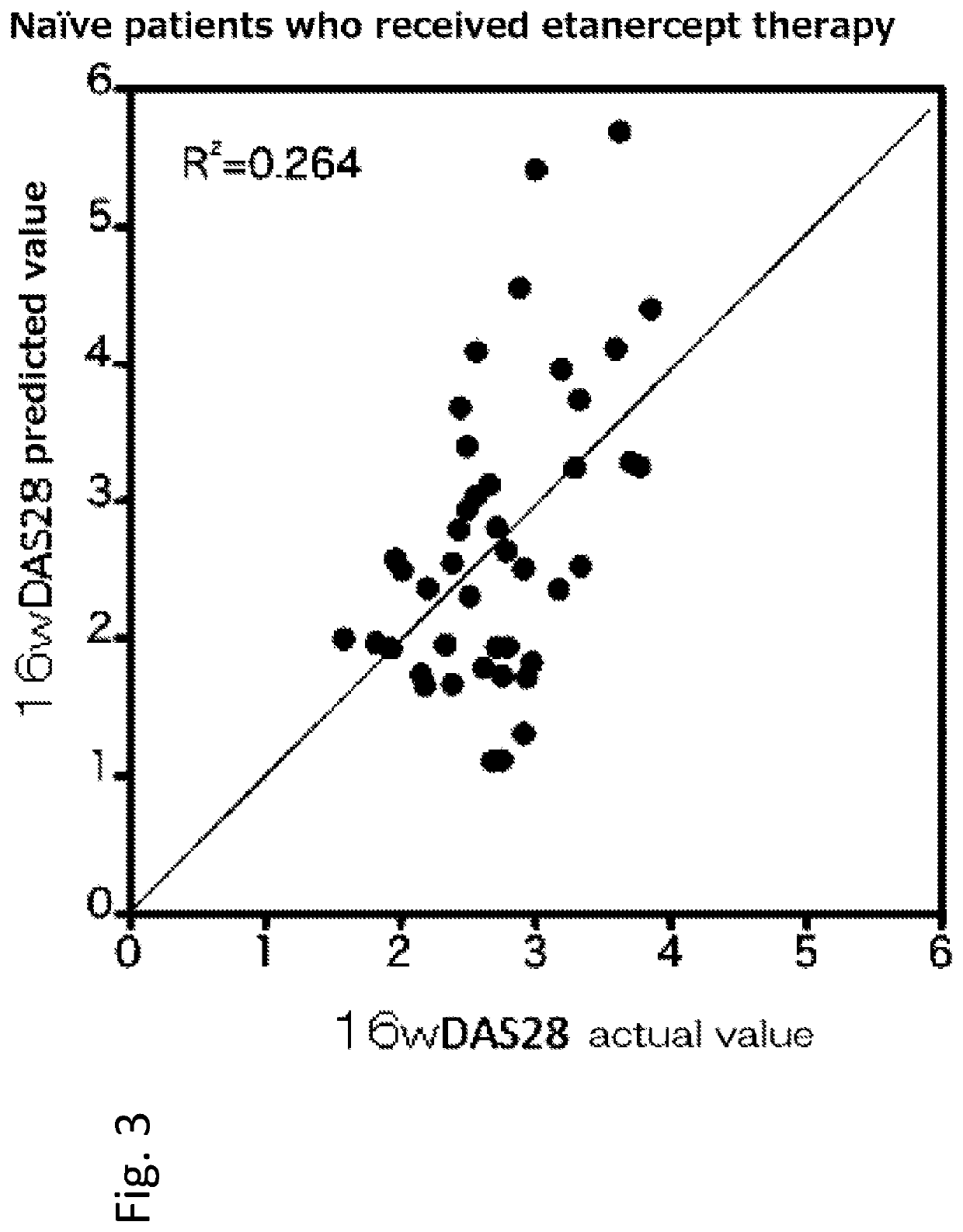
[0169]Hereinafter, a rheumatism patient who has not received anti-cytokine therapy (administration of infliximab, etanercept, adalimumab, tocilizumab or the like) in the past is referred to as a naïve patient, and a rheumatism patient who has received anti-cytokine therapy in the past is referred to as a switch patient.
[0170]155 rheumatoid arthritis patients, to whom methotrexate therapy was ineffective, were registered at the Higashihiroshima Memorial Hospital from March 2008 to June 2013. Among the 155 patients, 98 patients received t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| anti-tumor necrosis factor-α | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com