USE OF 6-THIO-dG TO TREAT THERAPY-RESISTANT TELOMERASEPOSITIVE PEDIATRIC BRAIN TUMORS
a technology of telomerase and 6-thiodg, which is applied in the field of use of 6thiodg, methods and compositions for treating pediatric brain cancer, can solve the problems of rapid telomere regrowth, significant lag period, and inability to tolerate egimen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
and Methods
[0145]Cell Lines and Primary Tumor Cell Culture.
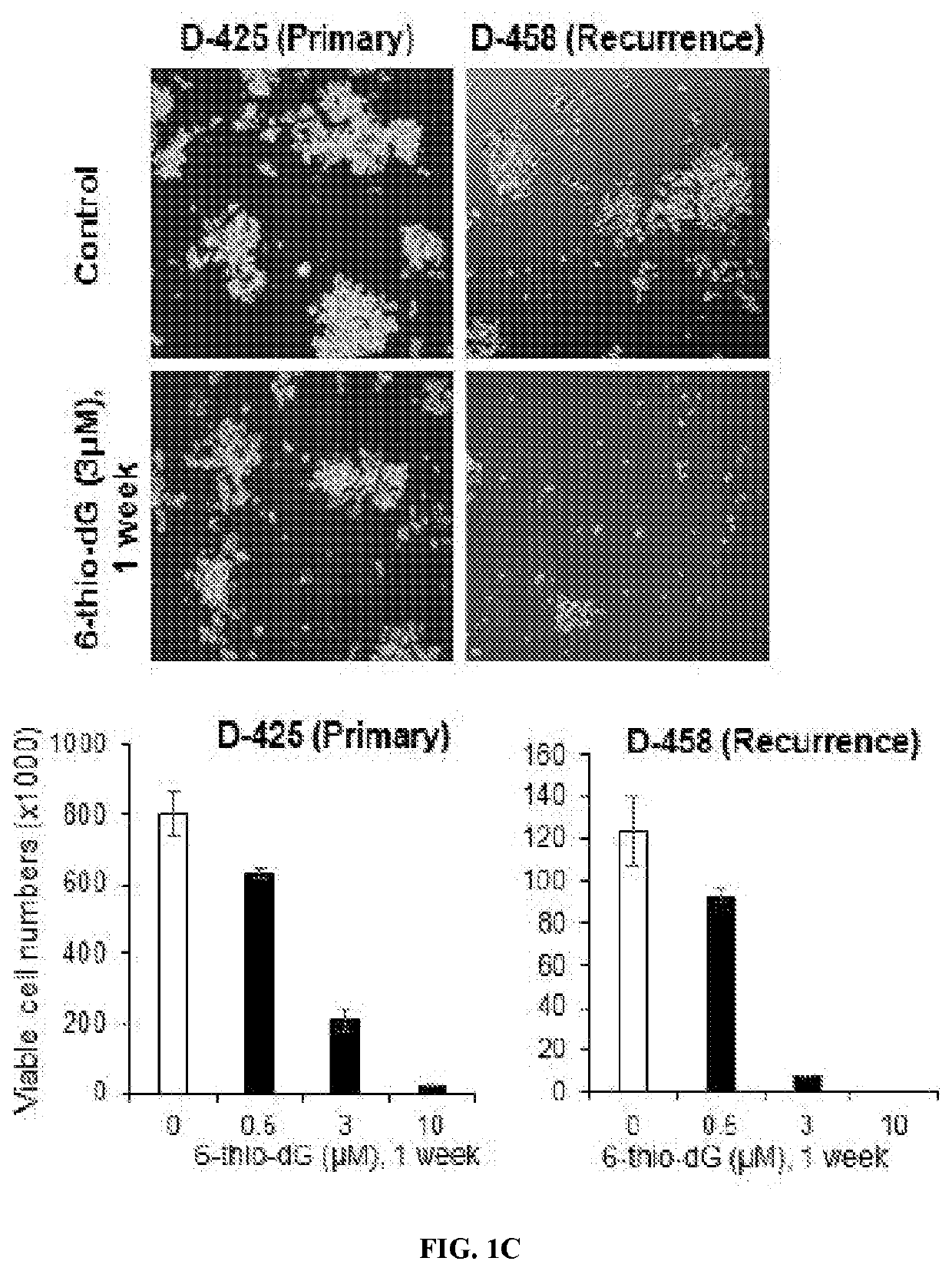
[0146]All patient specimens were collected after obtaining written informed consent from patients and families in accordance with approved IRB studies. The primary DIPG neurosphere line CCHMCDIPG-1 was aseptically isolated by dissociating the brain tumor tissue post-autopsy from a patient consented under the Pediatric Brain Tumor Repository (PBTR) study (IRB approved protocols 2013-1245 and 2013-5947) at Cincinnati Children's Hospital Medical center (CCHMC). Primary patient-derived neurospheres high-risk group-3 MB (MB004) (19,20), GBM (R0315-GBM), and DIPG)SU-DIPG-VI (21) and CCHMC-DIPG-1 (22)) were cultured in neurosphere stem cell media as described elsewhere (22,23). Patient-derived medulloblastoma cell lines collected from the same patient at diagnosis D-425, and at recurrence D-458 (24,25) were cultured in RPMI-1640 (Gibco) supplemented with 15% FBS. Primary normal human foreskin fibroblast (HFF) strain (ATCC CRL-2091)...
example 2
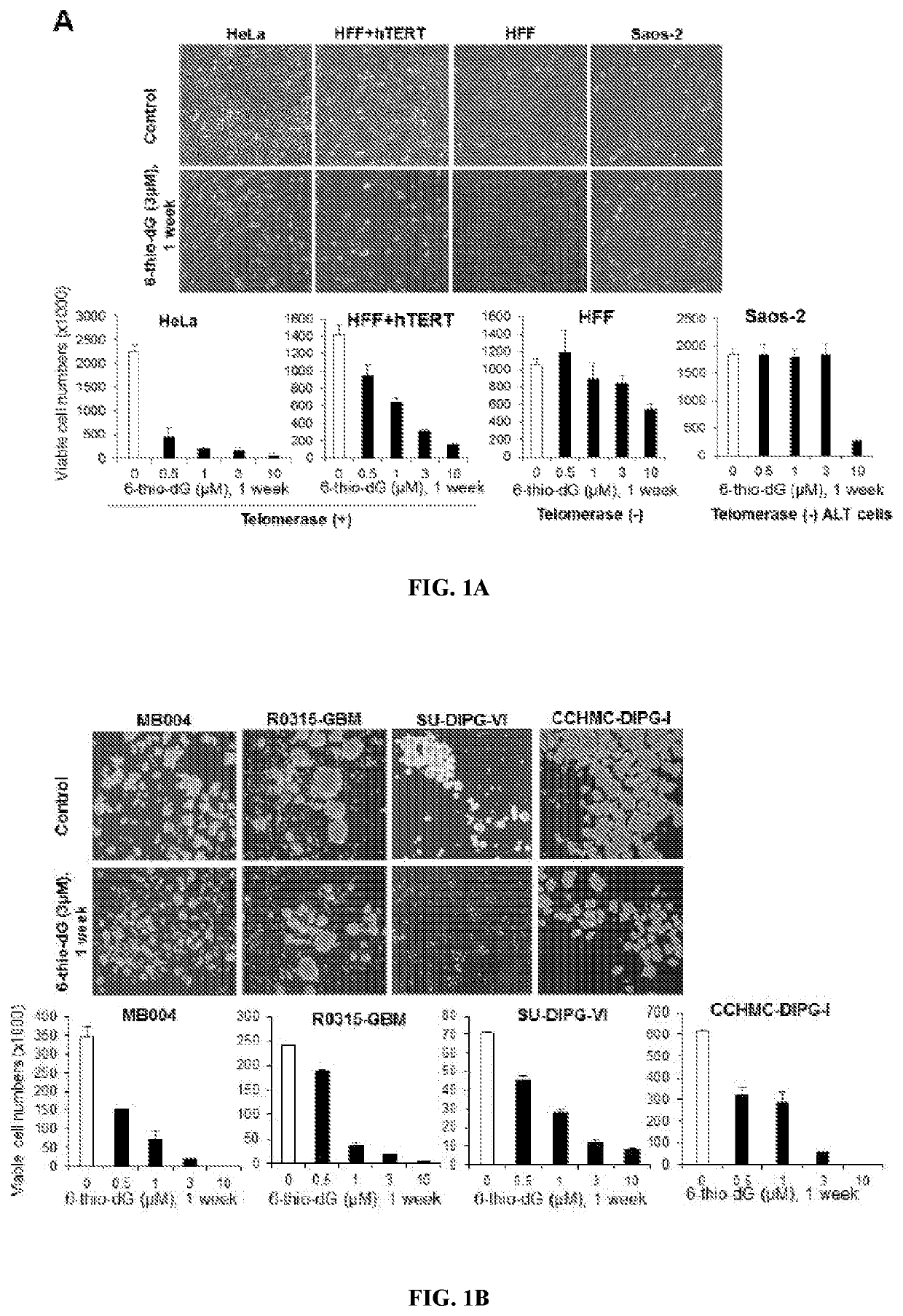
[0171]6-Thio-dG Selectively Inhibits Cell Growth of Telomerase-Positive Tumor Cells.
[0172]One of the major setbacks in oncology is the ability of certain cancers to recur after minimal or undetectable disease is achieved with aggressive therapies. Cancer stem-like cells have been proposed to represent a sub-population of cells within a tumor that self-renew to promote tumor growth and recurrence. In the present study, primary stem-like cells were derived from DIPG, HGG and MB patients' tumor tissue and expanded in neurosphere stem cell media. Table S1 indicate genetic features and subtypes of the cell lines. The inventors tested 6-thio-dG in a panel of telomerase-positive pediatric brain tumor cells, including high-risk group-3 MB (MB004), GBM (R0315-GBM), and DIPG (SU-DIPG-VI and CCHMC-DIPG-1) along with a panel of control cell lines, consisting of normal primary human foreskin fibroblasts (HFF, telomerase-negative), HFF-ectopically expressing hTERT (HFF+hTERT, telomerase-positive)...
example 3
n
[0186]Telomerase activity is present in most human cancers but is undetectable in the majority of normal human somatic cells, supporting the rationale of targeting telomerase and telomeres to treat cancer. The inventors' previous clinical trial of imetelstat, a potent direct inhibitor of telomerase, proved intolerable and ineffective in children with recurrent CNS malignancies. The inventors believe that this was due, at least in part, to toxicities, such as thrombocytopenia, which led to CNS bleeding that prevented more frequent dosing of imetelstat to allow sustained inhibition of telomerase. Mender et al. reported that no animal deaths or weight loss were observed in mice treated with 6-thio-dG. Moreover, the treatment did not cause any toxic effects on hematologic counts, liver and kidney functions (17).
[0187]Using a lung cancer model, the previous study has demonstrated that 6-thio-dG caused both, in vitro and in vivo telomere damage (TIFs) and induced rapid cancer cell death,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com