Modified gamma delta t cells and uses thereof
a technology of gamma delta t cells, which is applied in the field of modified, can solve the problems that studies have not realized the potential to use car modified t cells from one subj
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
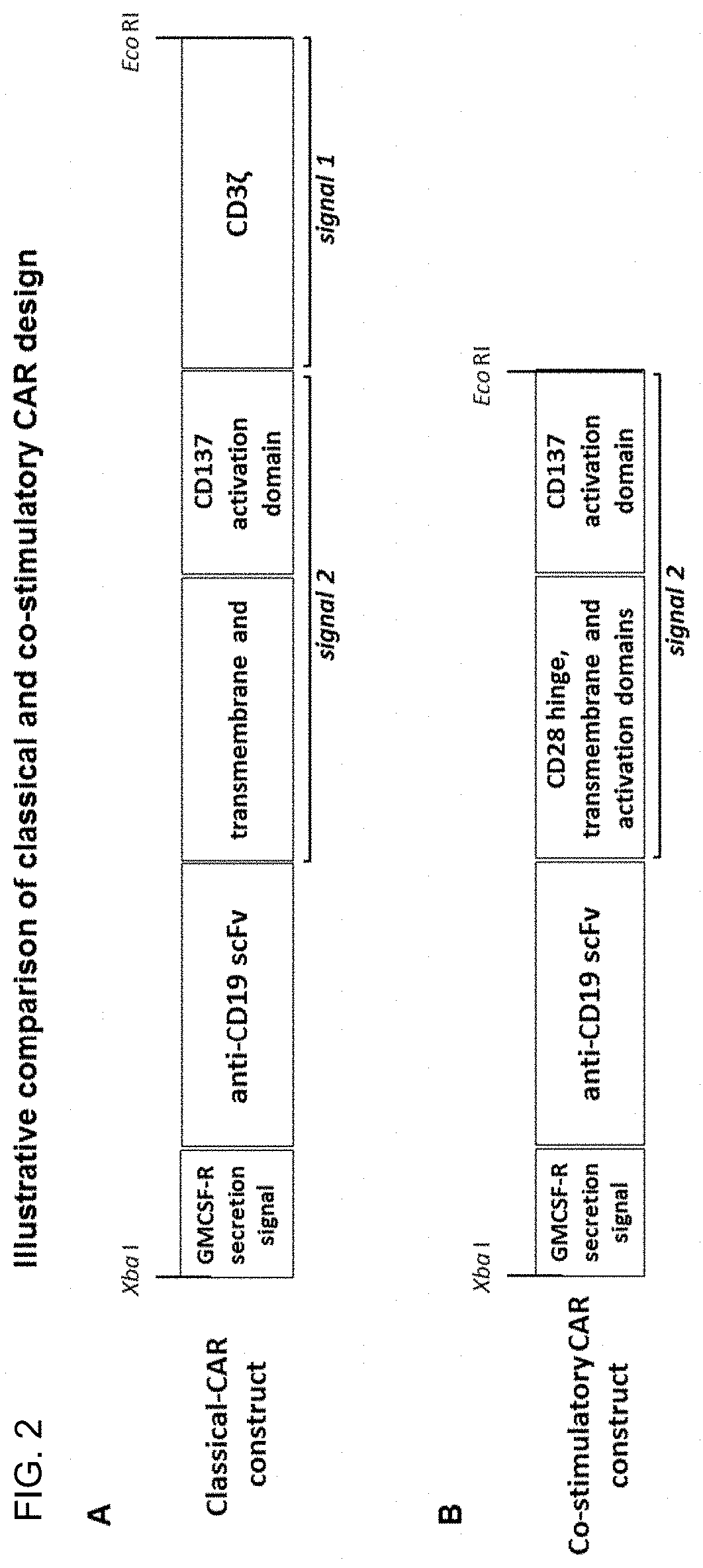
[0176]PBMCs were isolated by density centrifugation (lymphoprep) from leukapheresis material and cryopreserved. PBMCs were resuscitated and zoledronic acid (5 μM) stimulated PBMCs were cultured in the presence of IL-2 (1000 IU / mL) and 5% human AB serum in growth media. After 48 hours in culture (37° C., 5% CO2, humidified atmosphere), cells were transduced with lentivirus containing a lenti-CMV-MCS-EF1a-puro construct with either a classical CAR sequence (anti-CD19 scFv-CD28-CD137-CD3) or a co-stimulatory CAR sequence (anti-CD19 scFv-CD28-CD137) and 5 μg / mL polybrene at an MOI of 10. Transduction was repeated 24 hours later. CAR mRNA expression was verified by QPCR using universal primers which detected expression of both constructs at day 5. Specific expression of each construct was confirmed using a combination of the discriminatory primers and universal primers (as per FIGS. 5, 6).
example 2
[0177]Cells were transduced with lentivirus containing classical CAR sequence and expanded as described in example 1. 7 days following transduction, cytotoxic activity was assessed by co-culturing transduced or non-transduced γδT cells with CD19 positive target cell lines, Daudi or Ramos. Target cell lines were stained with the non-toxic membrane dye PKH67 (5 μM) for specific visualisation of the CD19 target population using flow cytometry. Following a 4.5 hour co-incubation with γδT cells or classical-CAR expressing γδT cells, co-cultures were stained with annexin V and propidium iodide (PI) to visualise apoptotic cells. The % specific cell death was calculated in the target cell population only. CAR-transduced γδT cells elicited increased potency in CD19 positive target cells in comparison to non-transduced γδT cells (FIG. 7).
example 3
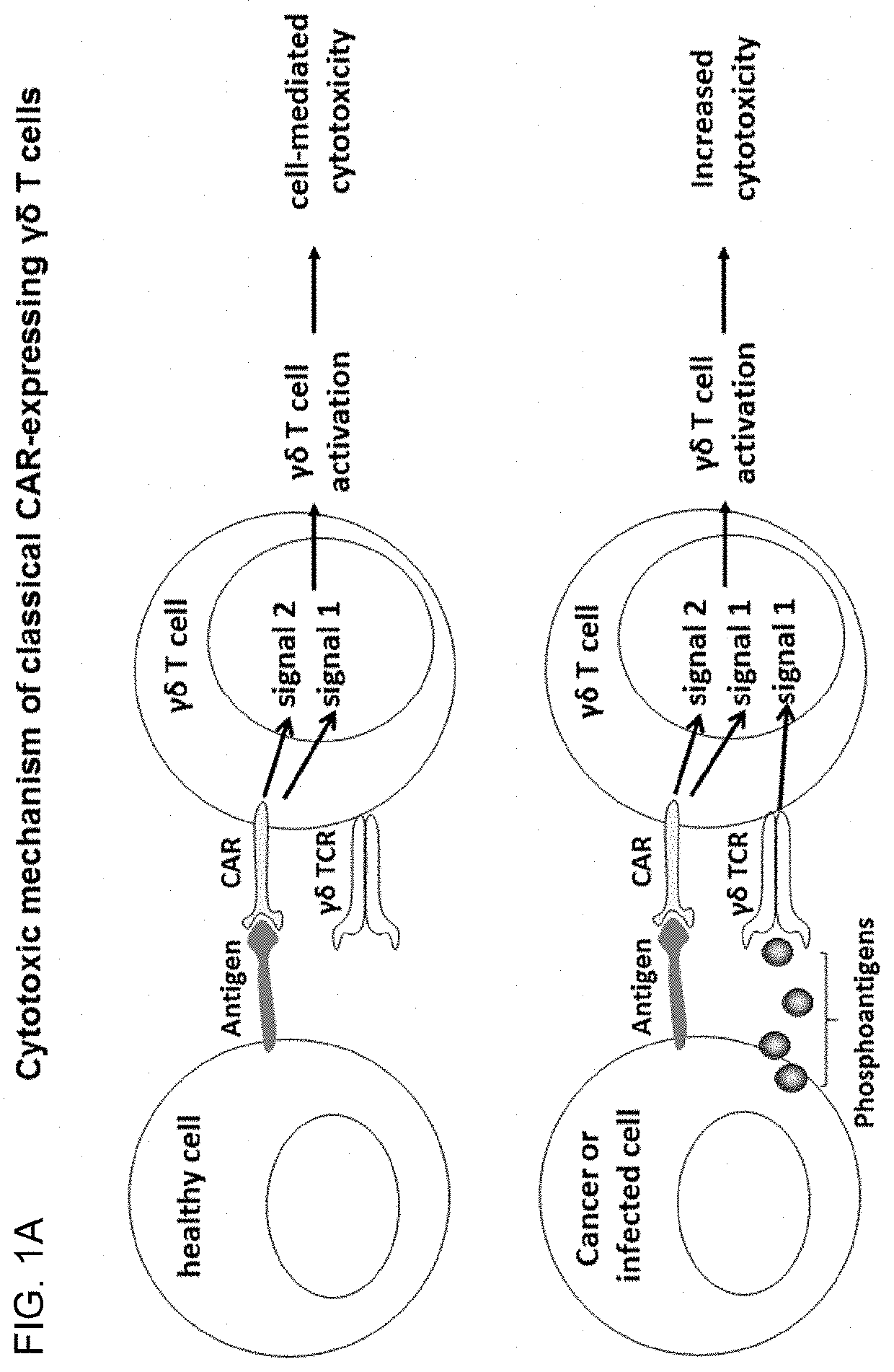
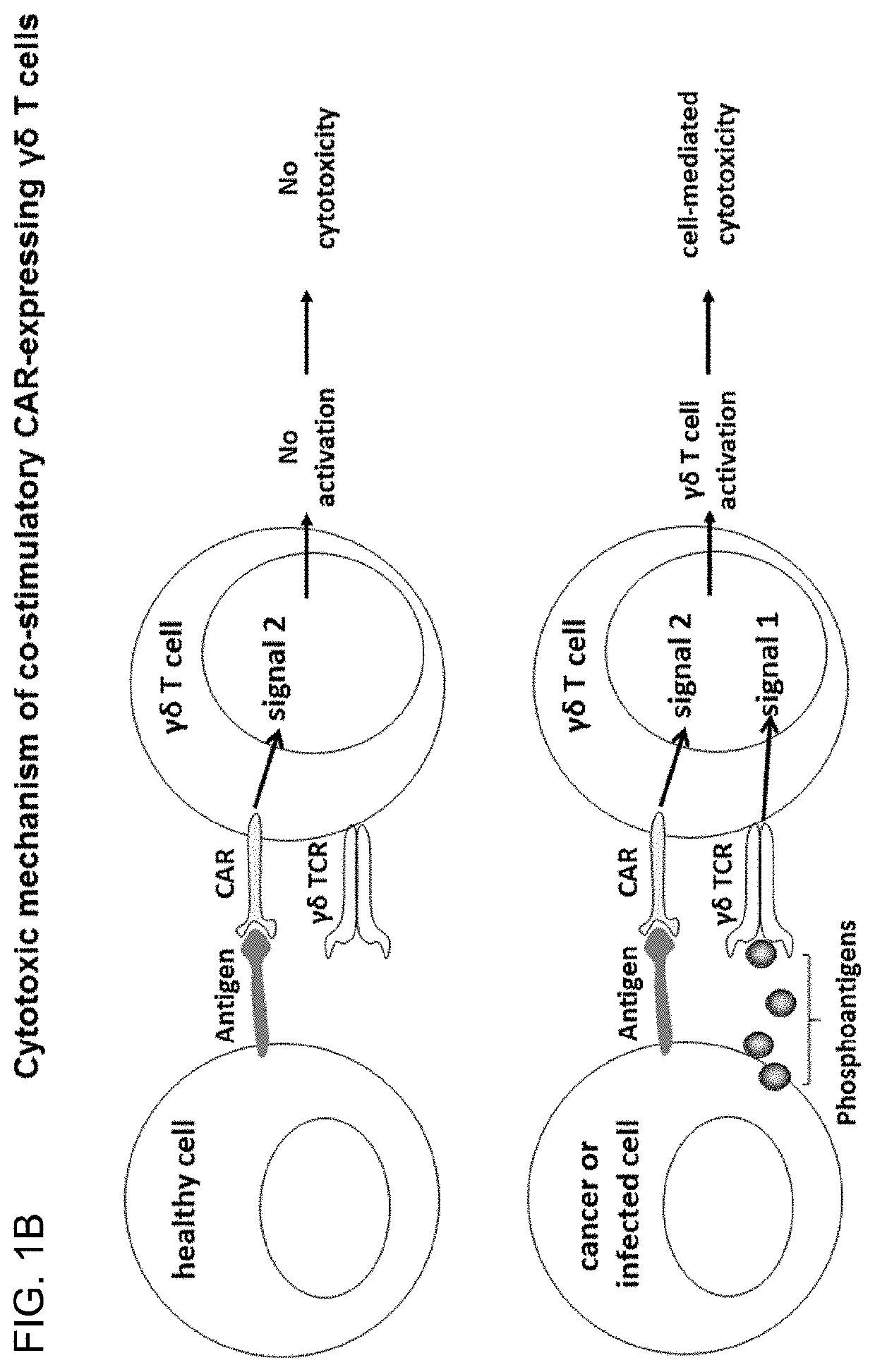
[0178]Cells were transduced with lentivirus containing classical CAR or costimulatory CAR sequence and expanded as described in example 1. The CD19 positive target cell lines Daudi and Ramos were pre-treated for 24 hours+ / −5 μM zoledronic acid. Cytotoxic activity was assessed as described in example 2. PKH67-stained target cells (+ / −zometa pre-treatment) were co-cultured with transduced or non-transduced γδT cells. The co-stimulatory CAR expressing γδT cells exhibited similar cytotoxicity towards the CD19 positive target cells as the classical-CAR expressing γδT cells, despite the absence of the CD3 activation domain, signal 1 instead provided via activation through the γδTCR by IPP sensing. Both CARs provided enhanced cytotoxicity towards γδT cells when compared to non-transduced γδT cells (FIG. 8).
[0179]Although the invention has been particularly shown and described with reference to particular examples, it will be understood by those skilled in the art that various changes in th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com