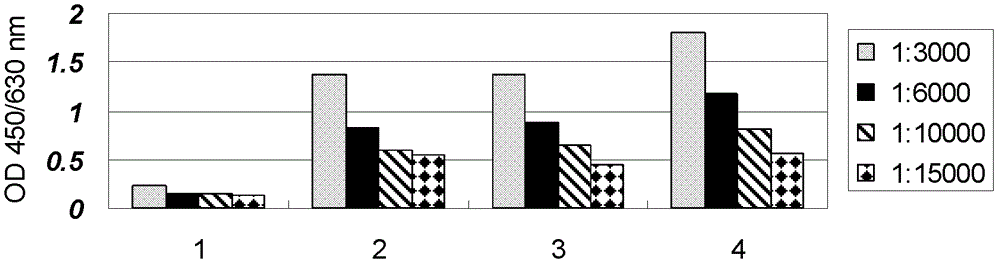
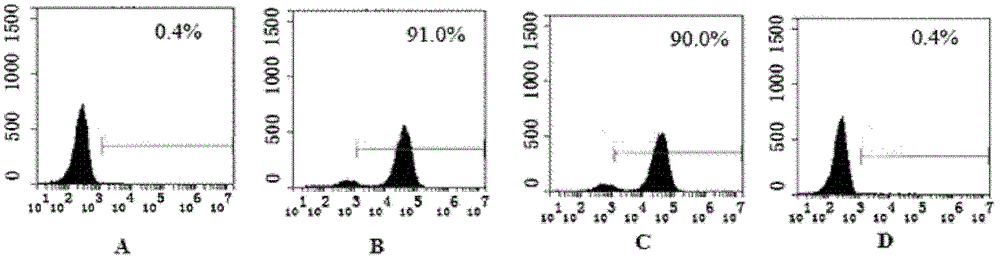
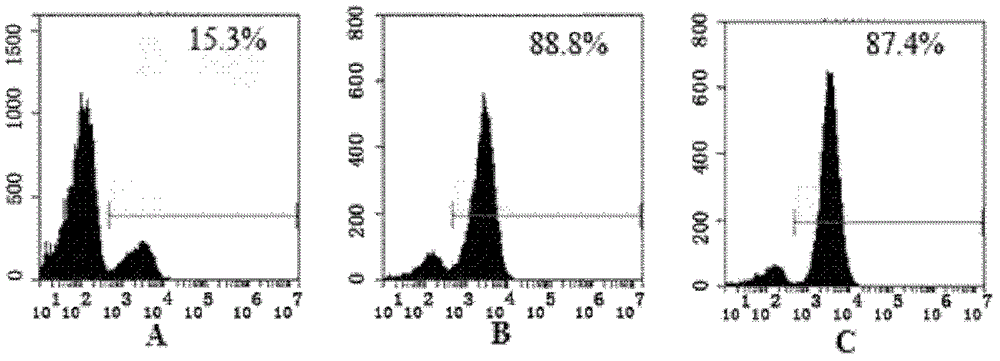
Recombinant single-chain antibody g5-4scfv of anti-human γδtcr monoclonal antibody and its encoding gene and application
A kind of technology of monoclonal antibody and single chain antibody
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1. Obtaining the recombinant single-chain antibody G5-4ScFv of anti-human γδTCR monoclonal antibody and its encoding gene
[0060] (1) Acquisition of anti-human γδTCR monoclonal antibody
[0061] The anti-human γδTCR monoclonal antibody of the present invention can be prepared according to conventional methods known in the art (as prepared by referring to the methods recorded in the following documents: Kohler and Milrtein, Nature 256: 495-96, 1975; Harlow and Lane, Aatibodies: A Laboratory Manual, Cold Spring Harbor Laboratory, 1988); the corresponding humanized anti-human γδTCR monoclonal antibody can also be prepared according to the method described in US Patent No. 5,585,089. The following description only provides a specific operation for the purpose of disclosure, and is not intended to limit the present invention.
[0062] 1. Animal immunity
[0063] 1. Primary immunization
[0064] 100 μg recombinant γ9δ2(OT3)-Fc ("Preliminary Identification of Expre...
Embodiment 2
[0140] Example 2. Expression and purification of recombinant single-chain antibody G5-4ScFv
[0141] 1. The expression vector of recombinant single-chain antibody G5-4ScFv and the construction of engineering bacteria
[0142] Take the recombinant plasmid inserted into the recombinant gene G5-4ScFv fragment constructed in Example 1 and have the correct sequence, carry out double digestion with BamH I / EcoR I or NdeI / EcoR I respectively, reclaim the double digestion product of 750bp, and separate the recovered fragments with The pET22b(+) vectors digested by BamH I / EcoR I or NdeI / EcoR I were ligated to obtain the expression vectors of recombinant single-chain antibody G5-4ScFv, which were named G5-4ScFv-PET22b(+)-sp and G5- 4ScFv-PET22b(+), double enzyme digestion results are as follows Figure 6 Shown (1, Marker; 2, before BamH I / EcoRI double digestion; 3, after BamH I / EcoR I double digestion; 4, after Nde I / EcoR I double digestion; 5, Nde I / EcoRI double Before enzyme digestio...
Embodiment 3
[0166] Example 3, Identification of recombinant single-chain antibody G5-4ScFv
[0167] The recombinant single-chain antibody G5-4ScFv obtained in Example 2 was identified.
[0168] 1. Physical and chemical properties
[0169] Mass spectrometry analysis of the molecular weight of the recombinant single-chain antibody G5-4ScFv, the mass spectrogram is as follows Figure 9 As shown, the molecular weight of the recombinant single-chain antibody G5-4ScFv is 29840.9kD.
[0170] 2. Identification by SDS-PAGE and Western blot
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com