Pharmaceutical composition for preventing or treating cancer comprising ksp inhibitor and mitosis inhibitor
a technology of mitosis inhibitor and pharmaceutical composition, which is applied in the direction of drug compositions, genetic material ingredients, biochemistry apparatus and processes, etc., can solve the problems of unreported research, the anti-cancer drugs inhibiting ksp cannot obtain significant clinical trials results, and the apoptosis of cancer cells, so as to achieve synergistic effects on anti-cancer activity, inhibit the expression of ksp, and facilitate the effect of anti-cancer activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Manufacture of Anti-Cancer Preparation Containing KSP siRNA and Paclitaxel
example 1-1
Manufacture of KSP siRNA-Containing Preparation
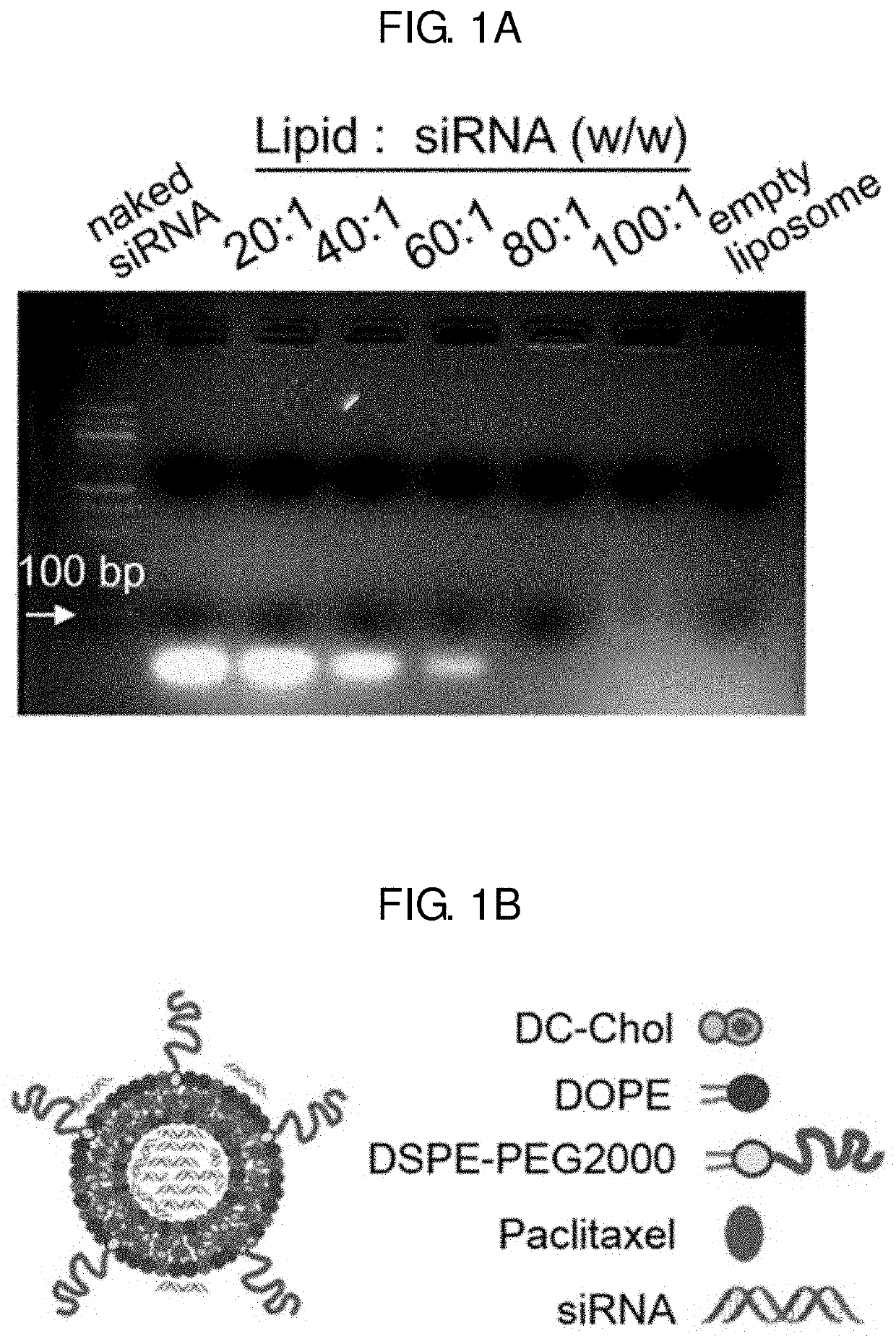
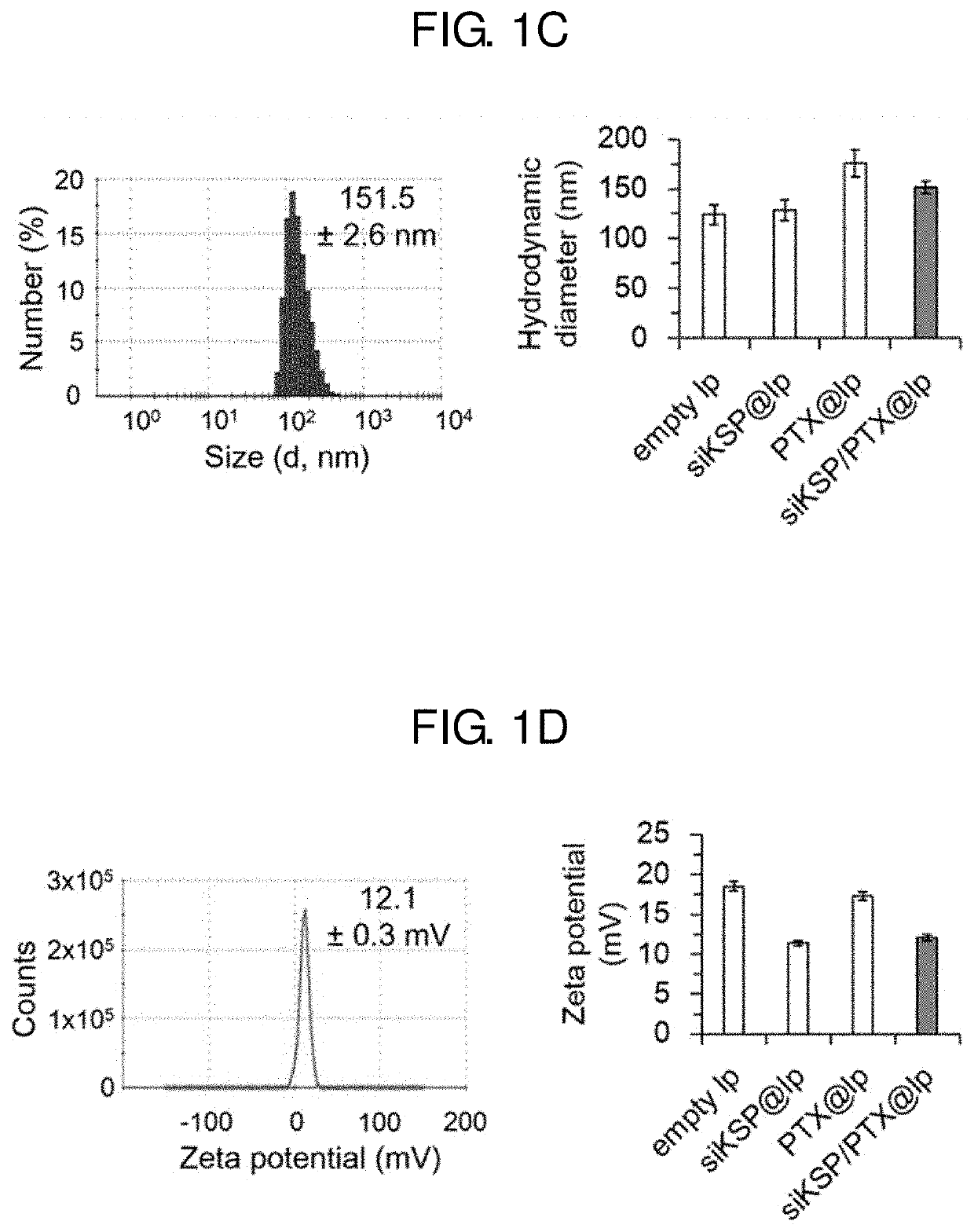
[0093]First, a PEGylated DC-Chol / DOPE cationic liposome was prepared using a known thin-film hydration method (Lee J, et al., Theragnosis 2016, 6, 192-203). DC-Chol, DOPE, and mPEG2000-DSPE were mixed in a ratio of 48.75:48.75:2.5 (molar ratio) such that a total weight of the mixture was approximately 300 μg, and chloroform was removed therefrom by lyophilization for 12 hours, thereby preparing a PEGylated liposome in the form of dry lipid film.
[0094]Subsequently, a PEGylated liposome into which KSP siRNA is introduced was prepared. Approximately, KSP siRNA consisting of nucleotides of SEQ ID NOS: 1 and 2 and a PBS buffer (containing 5% glucose, 10 mM, pH 7.4) were added to the PEGylated liposome in the dry lipid film form, followed by sonication for 30 seconds and stirring at room temperature for 4 hours to obtain a mixture. Herein, a mixing ratio of the PEGylated liposome and KSP siRNA was set in various ranges (20:1, 40:1, 60:1, 80:1...
example 1-2
Manufacture of Paclitaxel-Containing Preparation
[0100]A PEGylated liposome was prepared using the known thin-film hydration method (Lee J, et al., Theragnosis 2016, 6, 192-203). DC-Chol, DOPE, and mPEG2000-DSPE were mixed in a ratio of 48.75:48.75:2.5 (molar ratio) such that a total weight of the mixture was approximately 300 μg to obtain a lipid mixture, and paclitaxel was added to the obtained lipid mixture, thereby obtaining a final mixture. Chloroform was removed from the obtained final mixture by lyophilization for 12 hours to prepare a paclitaxel-containing PEGylated liposome.
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com