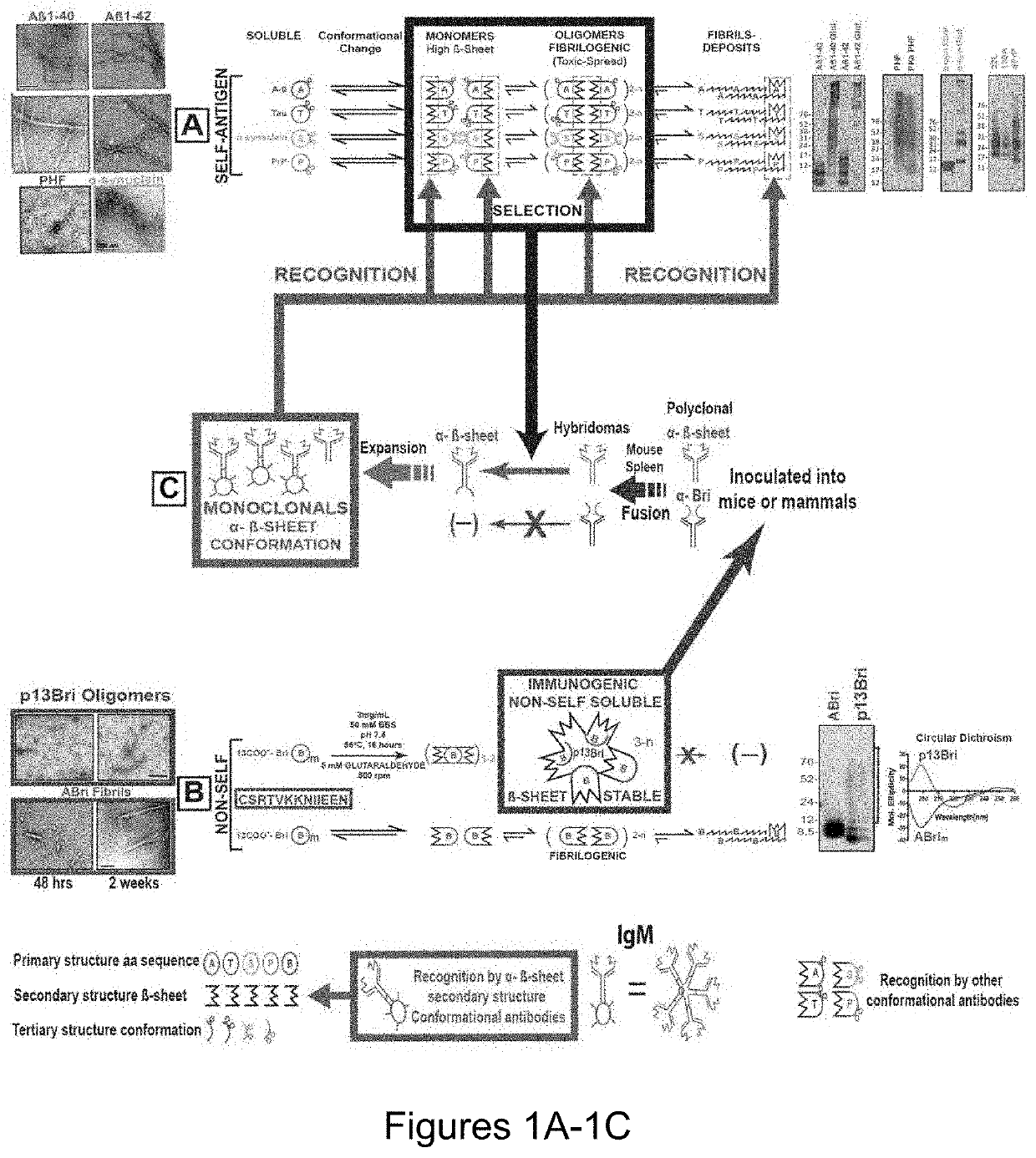
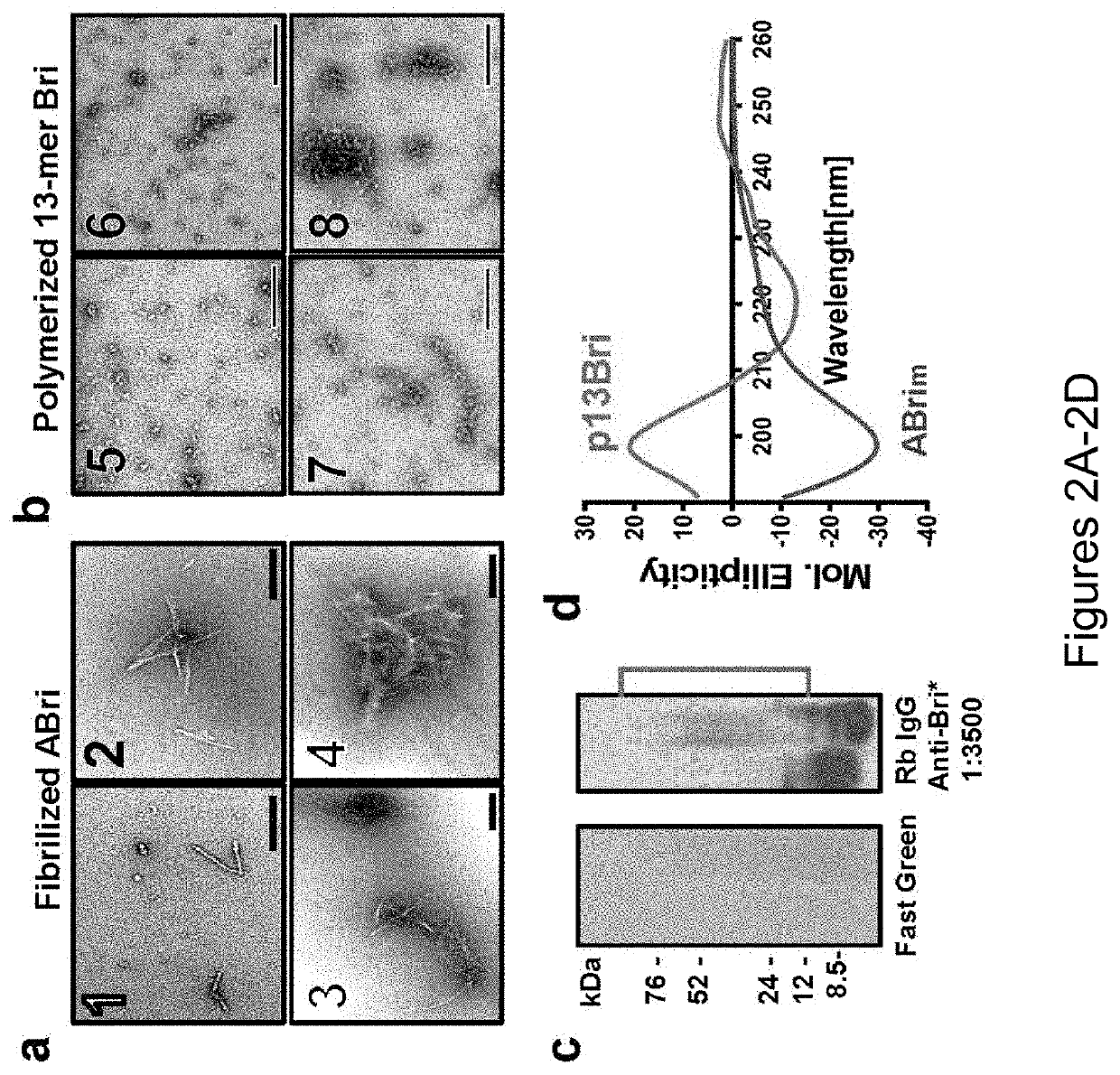
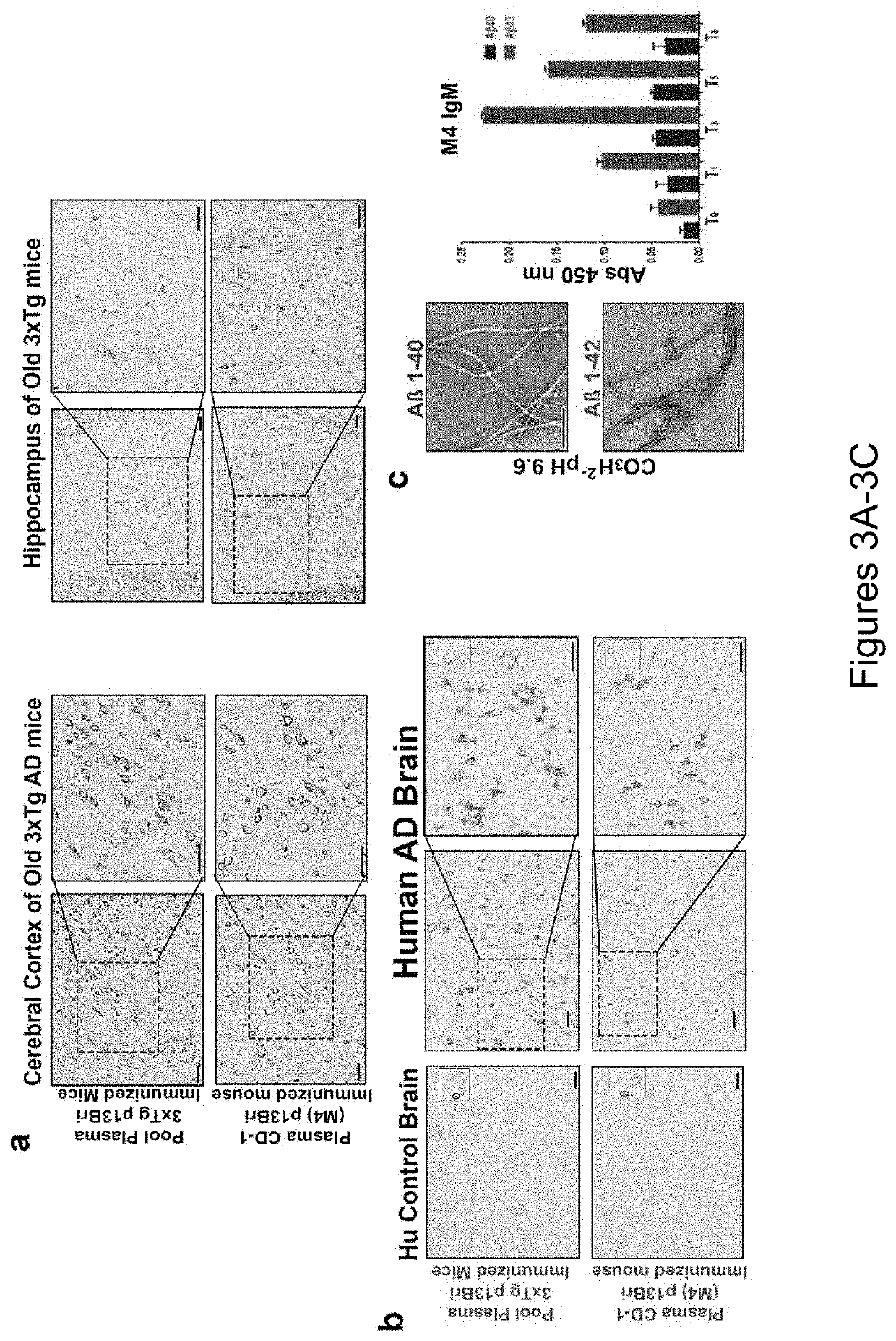
Specific murine and humanized monoclonal antibodies detecting pathology associated secondary structure changes in proteins and peptides
a monoclonal antibody and protein technology, applied in the field of antibodies and binding fragments thereof, can solve the problems of cell dysfunction, cell death, and fibrillary structure that becomes, and achieve the effects of reducing pathology, effective detection, monitoring and treating ndd, and reducing pathology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
n of Monoclonal Antibodies Against Conformational β-Sheet Secondary Structures Common to Oligomeric Forms of Different Pathological Peptides and Proteins of Various Neurodegenerative Diseases
[0215]Synthesis and Polymerization of 13-mer Bri Peptide (pBri). The procedure was performed as previously published (Goñi et al, “Immunomodulation Targeting Abnormal Conformation Reduces Pathology in a Mouse Model of Alzheimer's Disease,” PLoS One 5(10): e13391 (2010), which is hereby incorporated by reference in its entirety). Briefly, the 13 residue peptide that corresponds to the carboxyl terminus of ABri (Cys-Ser-Arg-Thr-Val-Lys-Lys-Asn-Ile-Ile-Glu-Glu-Asn; SEQ ID NO: 111) was synthetized on an ABI 430A peptide synthesizer (AME Bioscience, Chicago, Ill.) at the Keck peptide synthesis facility at Yale University, CT. Mass spectroscopy of the lyophilized end-product was used to verify the expected molecular weight.
[0216]To have a stable oligomeric conformation and make the 13 mer Bri peptide ...
example 2
ization of Anti-β-Sheet Conformational mAb aβComAb WG-3D7
[0253]The purification of the anti-β-sheet conformational monoclonal antibody aβComAb WG-3D7 is depicted in FIGS. 11A-11B. FIG. 11A shows western blots of the conformational monoclonal antibody WG-3D7 purified with a llama anti-μ column. The far left panel shows Fast Green staining for protein loading control, the middle panel shows anti-mouse IgM μ specific reactivity, and the right panel shows anti-mouse Kappa reactivity. Lanes 1 and 2 of each panel contain un-reduced and DTT reduced proteins, respectively (IgMk p=pentamer, Hμ r=reduced heavy chain, and Kr=Kappa light chain reduced). FIG. 11B contains graphs of ELISA data showing the reactivity of the purified conformational monoclonal antibody WG-3D7 against (in order from left to right on the graphs) Aβ40, oligomerized Aβ42, and PHF (left graph), and positive and negative controls showing that the antigen specificity of the WG-3D7 antibody (solid bars) is not an artifact o...
example 3
ization of Anti-β-Sheet Conformational mAb aβComAb FT-11F2
[0259]The purification of the anti-β-sheet conformational monoclonal antibody FT-11F2 with SAS and quantification of the antibody binding levels to pathological conformers is depicted in FIGS. 17A-17B. FIG. 17A shows Western blots of the conformational monoclonal antibody FT-11F2, which was initially purified using 50% SAS. The left panel shows Fast Green staining for protein loading, the middle panel shows anti-mouse IgM μ specific reactivity, and the right panel shows anti-mouse Kappa specific reactivity. Lane 1 contains un-reduced sample (IgMk p=pentamer) and lane 2 contains 0.1 M dithiothreitol (DTT) reduced sample (Hμ, r=reduced heavy chain and Kr=Kappa light chain reduced). FIG. 17B are graphs of ELISA data showing the reactivity of the purified conformational monoclonal antibody FT-11F2 against (in order from left to right on the graph) Aβ40, oligomerized Aβ42, PHF, and PrP (left graph). The right graph of FIG. 17B sho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com