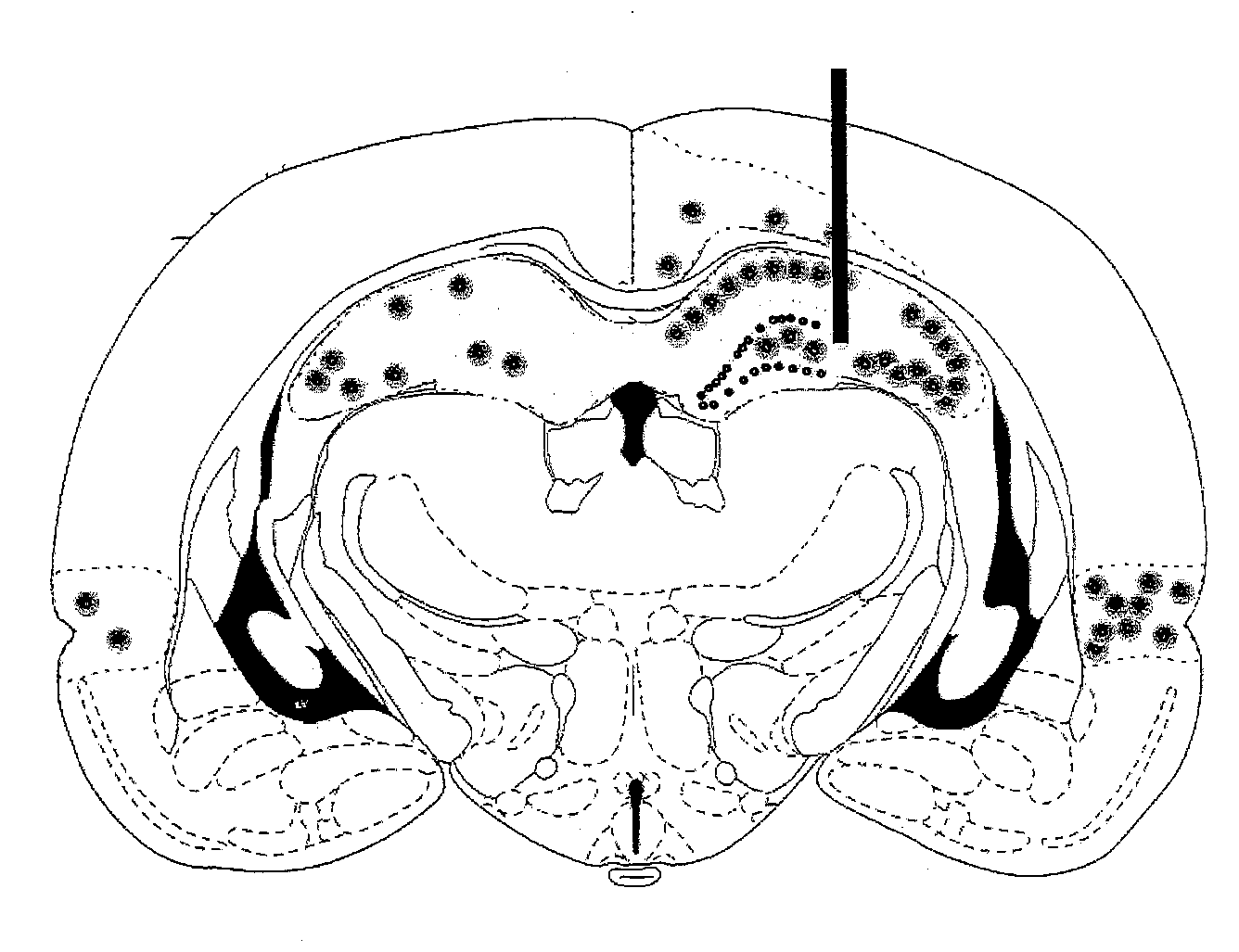
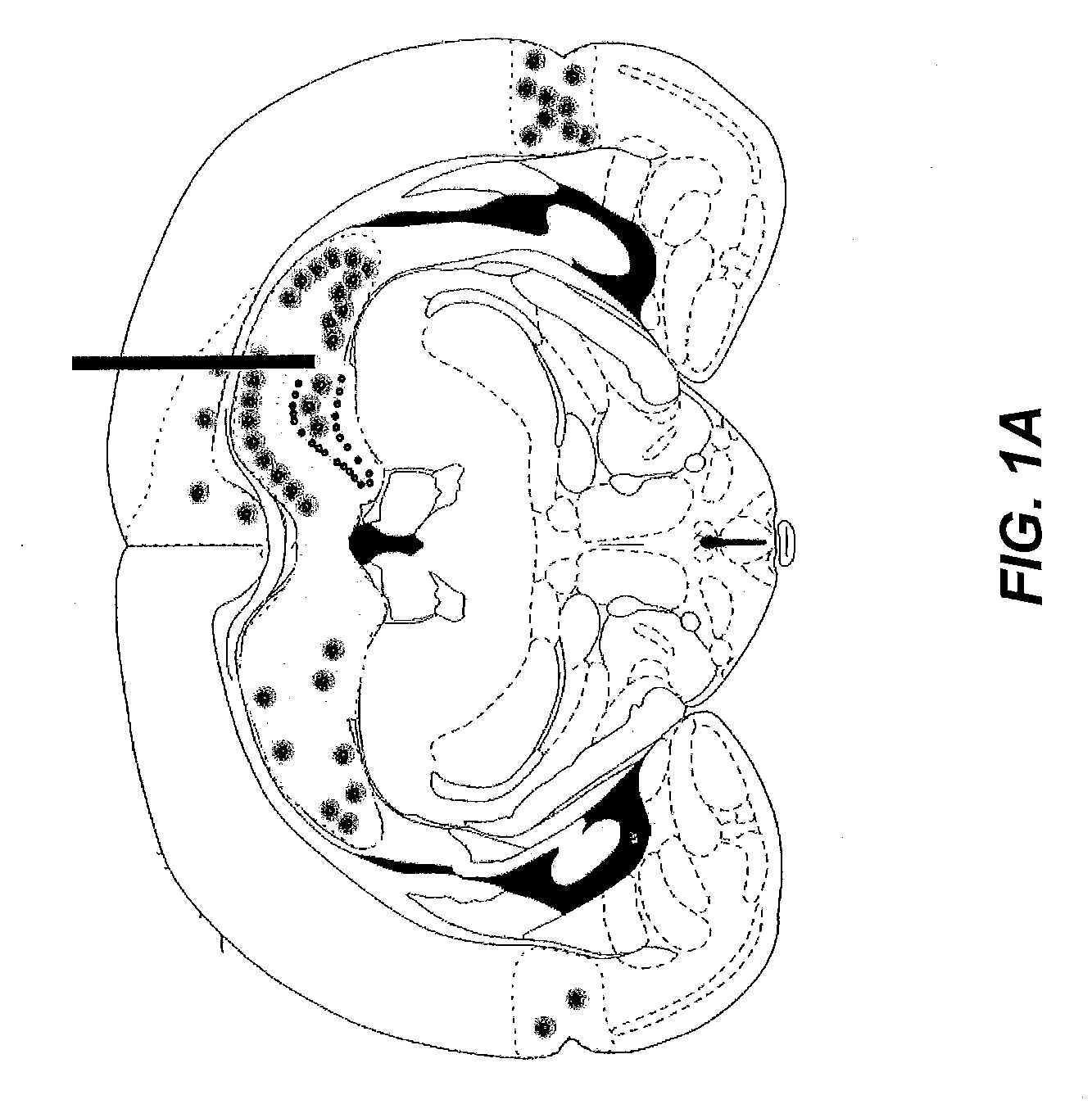
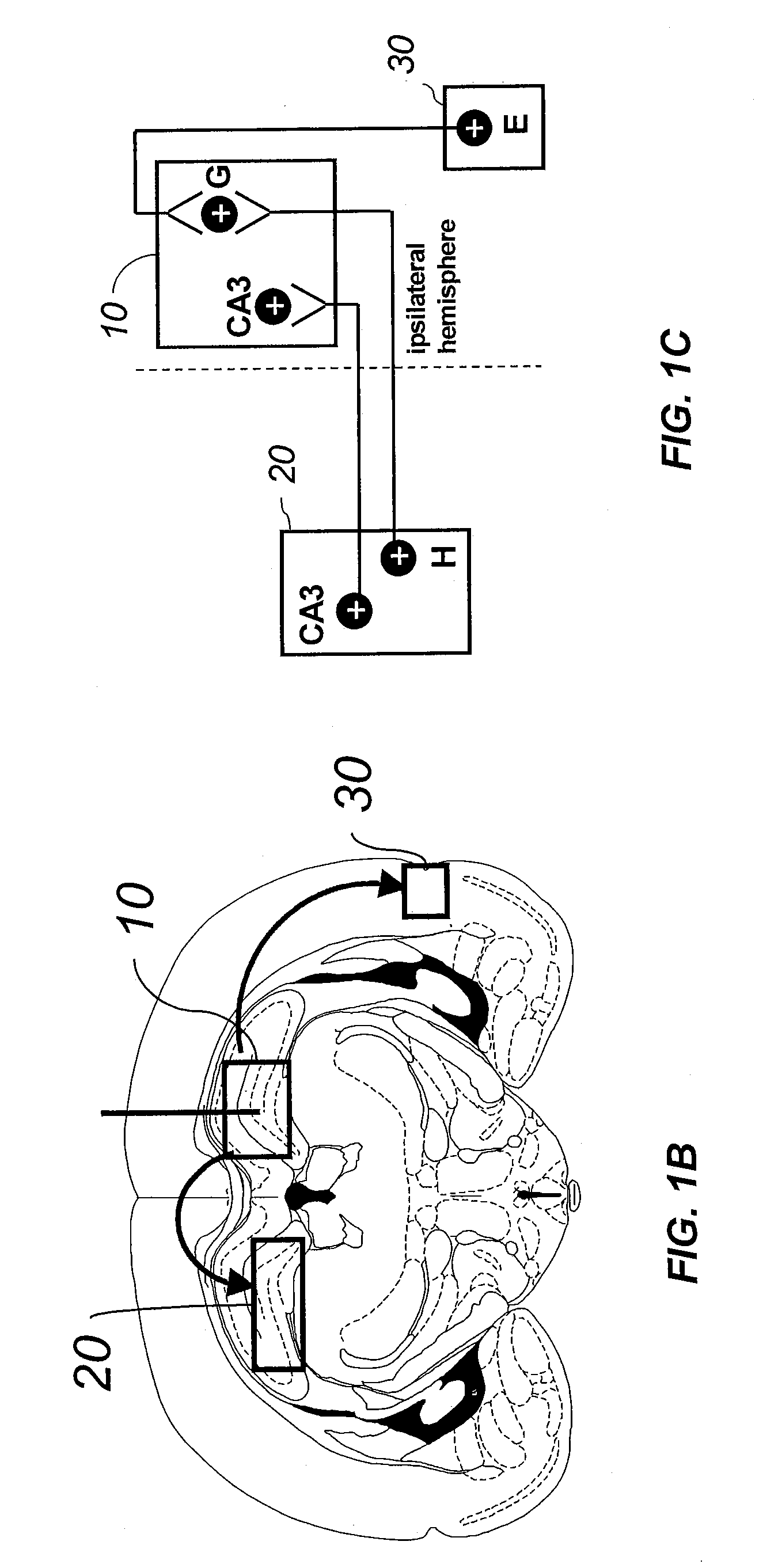
Gene therapy for spinal cord disorders
a technology for spinal cord disorders and gene therapy, applied in the field of gene therapy for spinal cord disorders, can solve the problems of not responding to intravenous ert, need to reverse lysosomal storage pathology in multiple separate tissues, and failure to introduce a replacement enzyme into the brain by direct injection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
Titration of Recombinant Vectors
[0076]AAV vector titers were measured according to genome copy number (genome particles per milliliter). Genome particle concentrations were based on Taqman® PCR of the vector DNA as previously reported (Clark et al. (1999) Hum. Gene Ther., 10:1031-1039; Veldwijk et al. (2002) Mol. Ther., 6:272-278). Briefly, purified AAV-ASM was treated with capsid digestion buffer (50 mM Tris-HCl pH 8.0, 1.0 mM EDTA, 0.5% SDS, 1.0 mg / ml proteinase K) at 50° C. for 1 hour to release vector DNA. DNA samples were put through a polymerase chain reaction (PCR) with primers that anneal to specific sequences in the vector DNA, such as the promoter region, transgene, or the poly A sequence. The PCR results were then quantified by a Real-time Taqman® software, such as that provided by the Perkin Elmer-Applied Biosystems (Foster City, Calif.) Prism 7700 Sequence Detector System.
[0077]Vectors carrying an assayable marker gene such as the β-galactosidase or green fluorescent pr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| distance | aaaaa | aaaaa |
| distance | aaaaa | aaaaa |
| distance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com