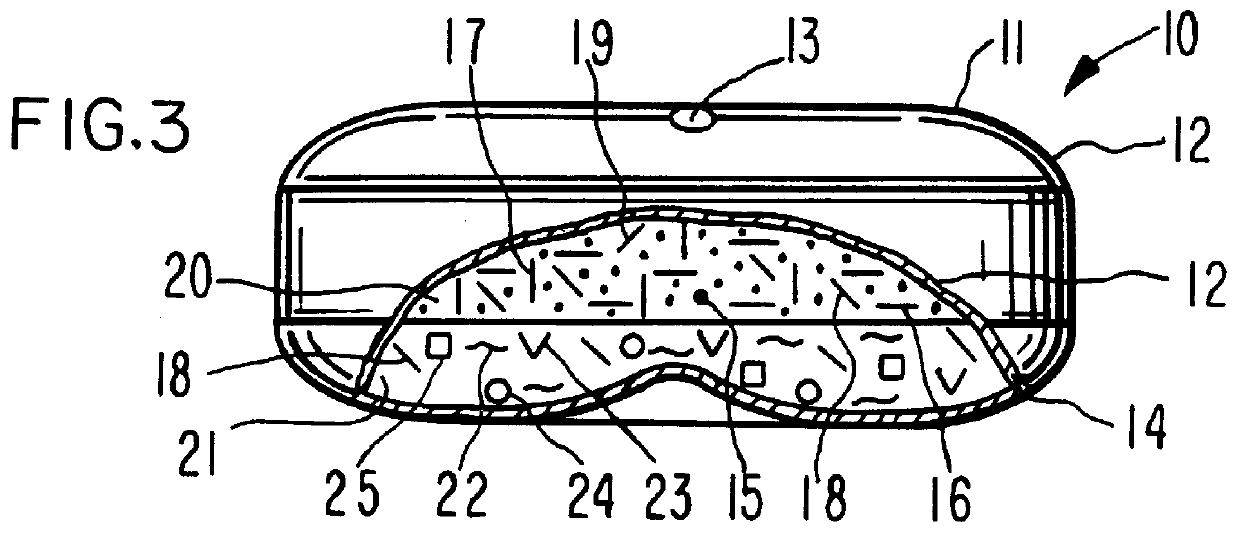
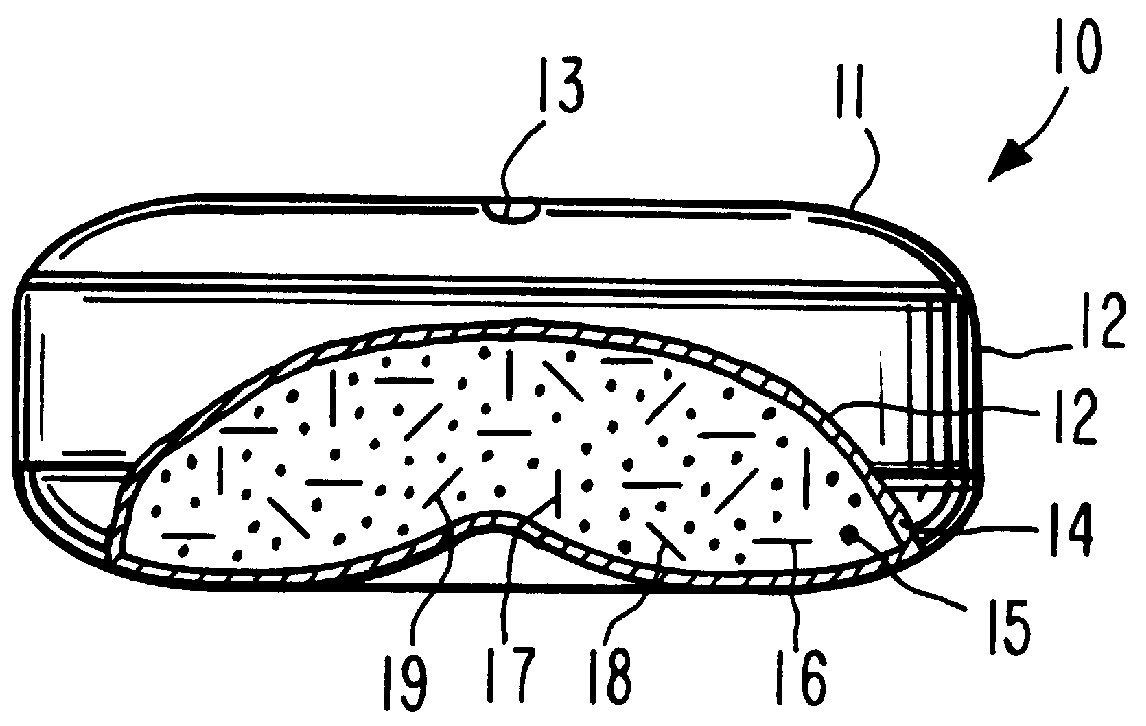
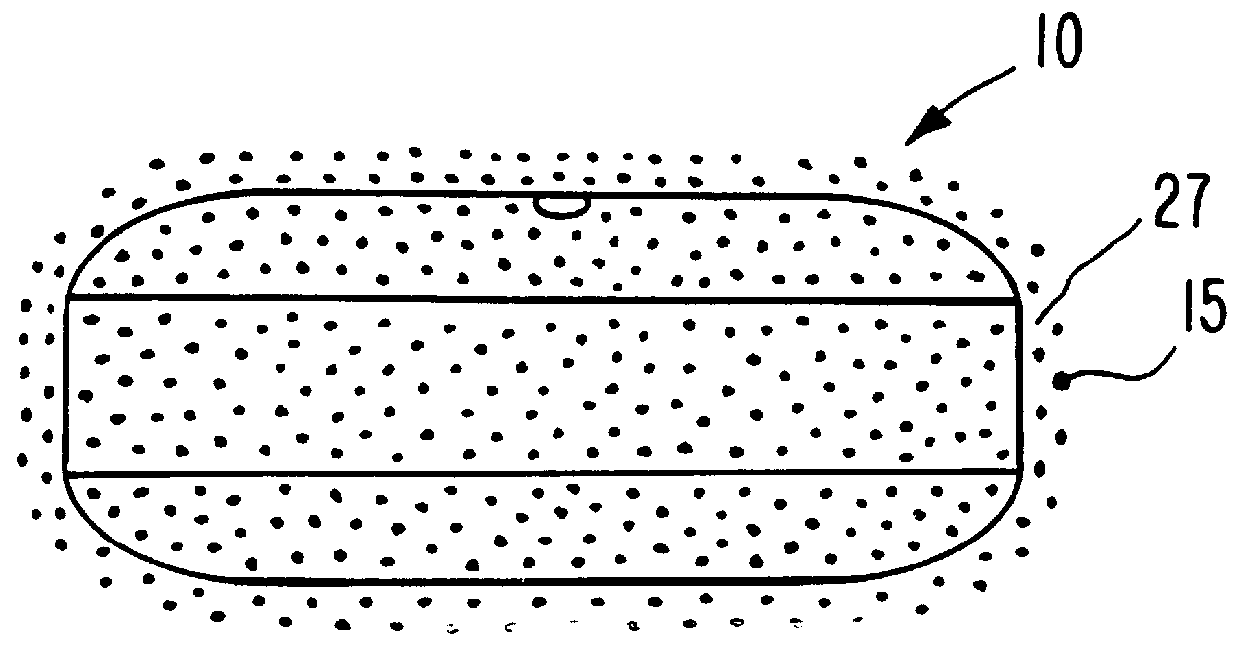
Therapy for neurological diseases
a neurological disease and therapy technology, applied in the field of neurological diseases, can solve the problems of uncontrolled rate therapy, drug tacrine, uncontrolled dosing of drugs, etc., and achieve the effect of slowing the progression of alzheimer's disease and avoiding the toxic range of tacrin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
A dosage form is manufactured for orally dispensing tacrine to the gastrointestinal tract of a human patient. The dosage form for dispensing tacrine to the gastrointestinal tract is unexpected, as tacrine has a low osmotic pressure that is substantially equivalent to the normal osmotic pressure of 8 plus atmospheres of the gastrointestinal tract. The osmotic pressure of the gastrointestinal tract is unpredictable and variable, attributed to diet, health and peristaltic mobility. Thus, a dosage form provided by this invention must develop an internal osmotic pressure greater than the osmotic pressure of the gastrointestinal tract, which, for this invention, is at least 10 atmospheres or higher in the dosage form, to provide a controlled rate of delivery of tacrine over a prolonged time. This invention effects an internal osmotic pressure of 10 atmospheres or more by blending tacrine, for example tacrine hydrochloride, with a fluid-imbibing, osmotically effective compound possessing a...
example 2
The dosage form of Example 1 is manufactured with a dose of tacrine coated on the exterior surface of the semipermeable wall. The dose of tacrine on the exterior wall comprises a pulsed dose of 15 mg of tacrine.
example 3
A dosage form adapted, designed and shaped as an osmotic tacrine dosage form is manufactured as follows: first, 3,290 g of tacrine hydrochloride and 3,290 g of mannitol are added to a Freund Flow-Coater bowl, a fluid bed granulator. The bowl is attached and the granulation process is initiated. Next, the dry materials are air suspended and mixed for 7 to 8 minutes. Then, a solution prepared by dissolving 175 g of poly(vinylpyrrolidone) having a molecular weight of 40,000 in 260 g of distilled water is sprayed onto the materials. The blending conditions are monitored during the process of spraying the aqueous poly(vinylpyrrolidone) at a solution spray rate of 125 g / min with an inlet temperature of 45.degree. C. and an air flow of 1,000 cfm. Next, the granules are blended with 35 g of hydroxypropylmethylcellulose and 210 mg of magnesium stearate and the granulation transferred to a Rotocone mixer and mixed to provide homogenous granules.
Next, a hydrogel expansion composition is prepar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com