Glycoside-containing liposome
a glycoside and liposome technology, applied in the field of liposomes, can solve the problems of insufficient antitumor effect, and achieve the effects of enhancing the solubility of a glycoside having antitumor activity, potent antitumor activity, and efficiently exhibiting its inherent antitumor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Formation of Liposomes from Cholestanol Glycoside
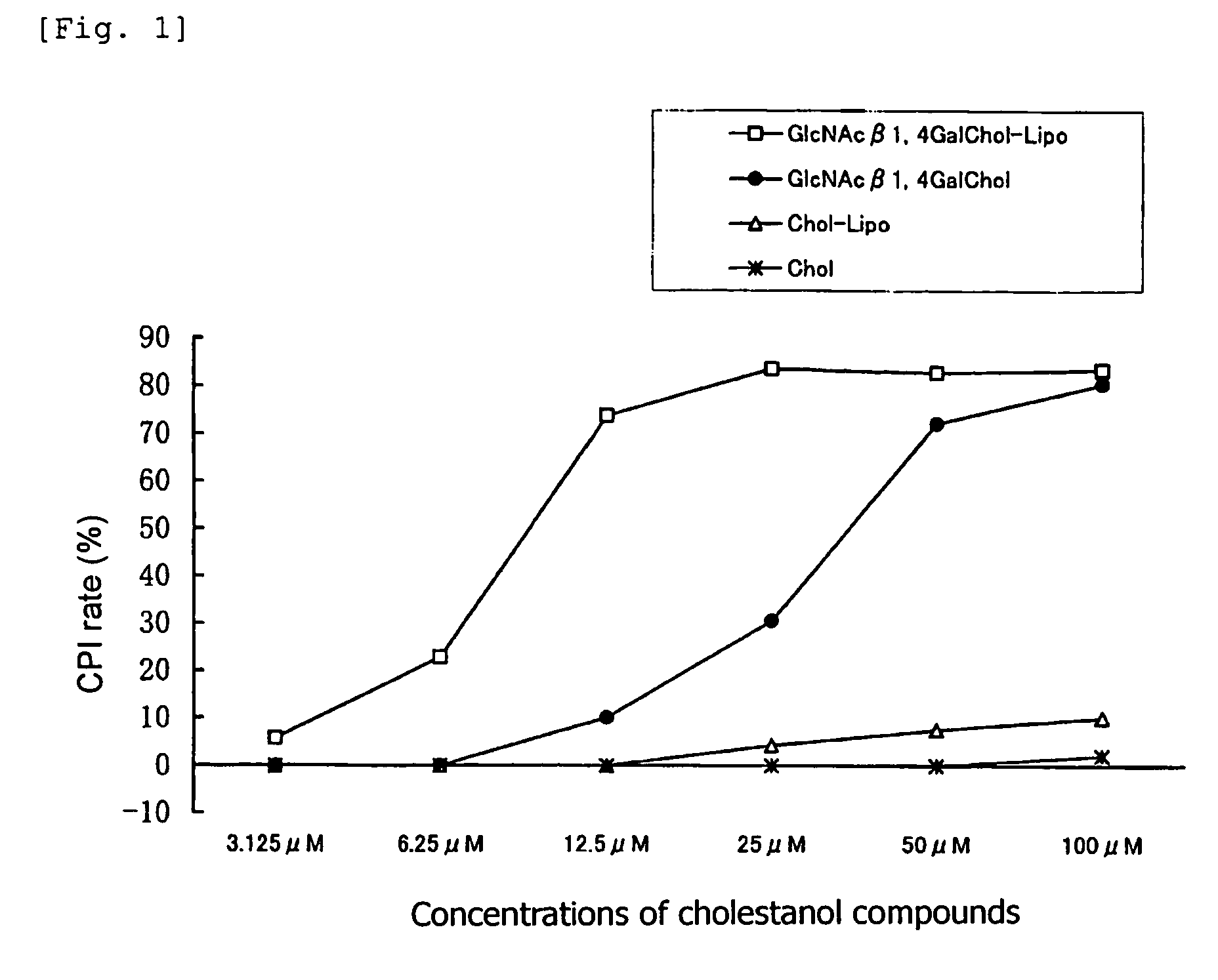
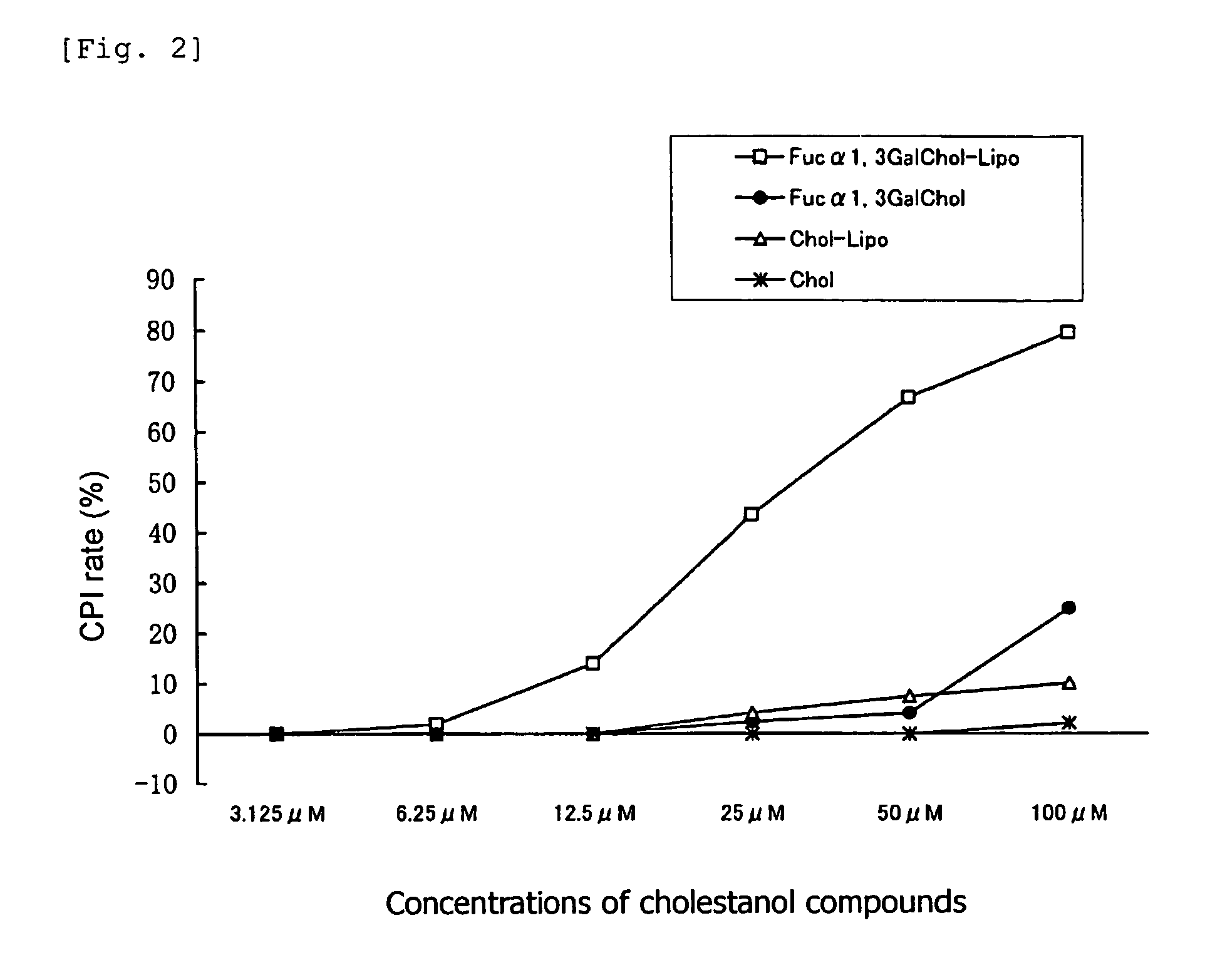
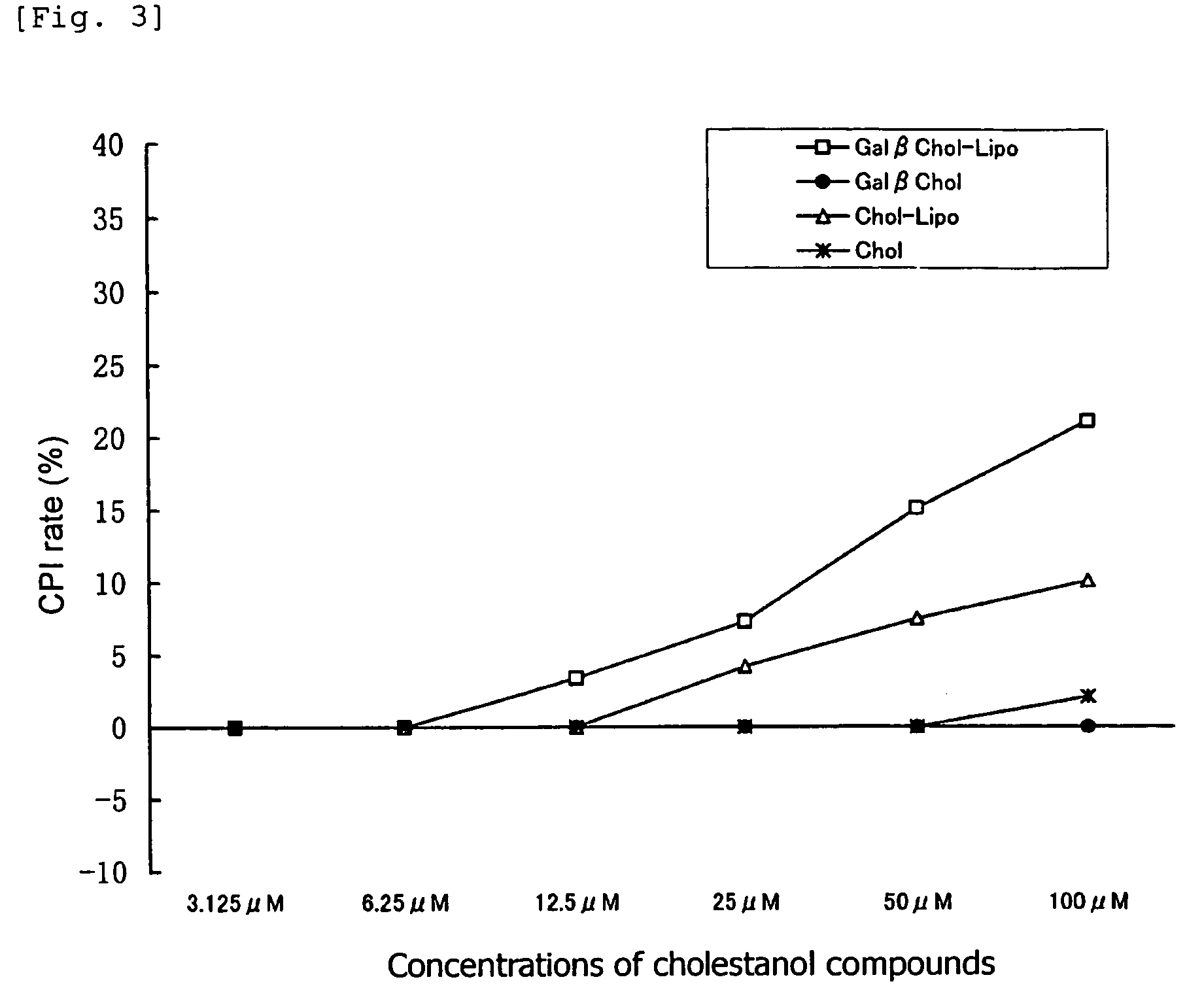
[0064]There were employed, as cholestanol glycosides, “GlcNAcβ1,4GalChol”; i.e., a cholestanol compound of formula (1) in which G is GlcNAcβ1,4Gal, “Fucα1,3GalChol”; i.e., a cholestanol compound of formula (1) in which G is Fucα1,3Gal, and “GalβChol”; i.e., a cholestanol compound of formula (1) in which G is Galβ. For comparison, “Chol”; i.e., a cholestanol compound of formula (1) in which G is H was employed. A 20 μmol / mL solution of each of these compounds (dissolved in chloroform / methanol=5 / 1 (v / v)) was employed as a starting material.
[0065]1α-Dipalmitoylphosphatidylcholine, stearylamine, and each of the aforementioned cholestanol compounds were mixed in proportions of 52 / 8 / 20 (by mole) so as to attain a total amount of 700 μL, and subsequently an organic solvent (chloroform / methanol=2 / 1 (v / v)) (300 μL) and distilled water (1 mL) were added to and mixed with the resultant mixture. Thereafter, the organic solvent was completely remo...
example 2
Formation of Liposomes from Naphthalene Methanol Glycoside
[0066]There was employed, as a naphthalene methanol glycoside, “GlcNAcβ1,4Galβ1,1NM” (NM: naphthalene methanol); i.e., a compound of formula (2) in which G is GlcNacβ1,4-Galβ1,1-, and liposomes were formed from this naphthalene methanol glycoside under conditions similar to those for formation of the cholestanol glycoside liposomes.
example 3
Formation of Liposomes from Ceramide Glycoside
[0067]There was employed, as a ceramide glycoside, “GlcNAcβ1,3Galβ1,4Glcβ1,1Ceramide”; i.e., a compound of formula (3) in which G is GlcNacβ1,3-Galβ1,4-Glc-, and liposomes were formed from this ceramide glycoside in a manner similar to that of formation of the cholestanol glycoside liposomes. In the case of formation of ceramide glycoside liposomes, cholesterol was added as a stabilizer.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com