CD70 inhibition for the treatment and prevention of inflammatory bowel disease
a technology of inflammatory bowel disease and inhibition of cd70, which is applied in the direction of antibody medical ingredients, drug compositions, peptides, etc., can solve the problems of disrupting the normal functioning of the bowel, and achieve the effect of reducing or eliminating the inflammatory condition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Methods
Mice
[0063]C57BL / 6 and BALB / c mice were purchased from the Jackson Laboratory (Bar Harbor, Me.). T-GFP Tg mice, backcrossed to C57BL / 6 mice for 7 generations, were bred into P14 TCR-Tg mice, specific for the LCMV gp33-41 peptide, that had been extensively backcrossed to C57BL / 6 mice (N10) to derive P14T-GFP mice. See Manjunath, N. et al., Proc. Natl. Acad. Sci. U.S.A., 96, 13932-13937 (1999), and Pircher, H. et al., Nature, 342, 559-561 (1989). LTα-deficient mice used herein are described in De Togni, P. et al., Science, 264, 703-707 (1994). All mice were maintained under specific pathogen-free (SPF) conditions in microisolater cages, and were used when they were 6-10 weeks of age.
Adoptive Transfer
[0064]Naïve CD8+ T cells were purified from splenocytes of P14 or P14T-GFP mice by negative selection using the murine T cell CD8 subset isolation kit (R&D systems, Minneapolis, Minn.) according to the manufacturer's instructions. The isolated cells were greater than 90...
example 2
Presence of an Unusual Type of Dendritic-like Cells in the Intestinal Mucosa
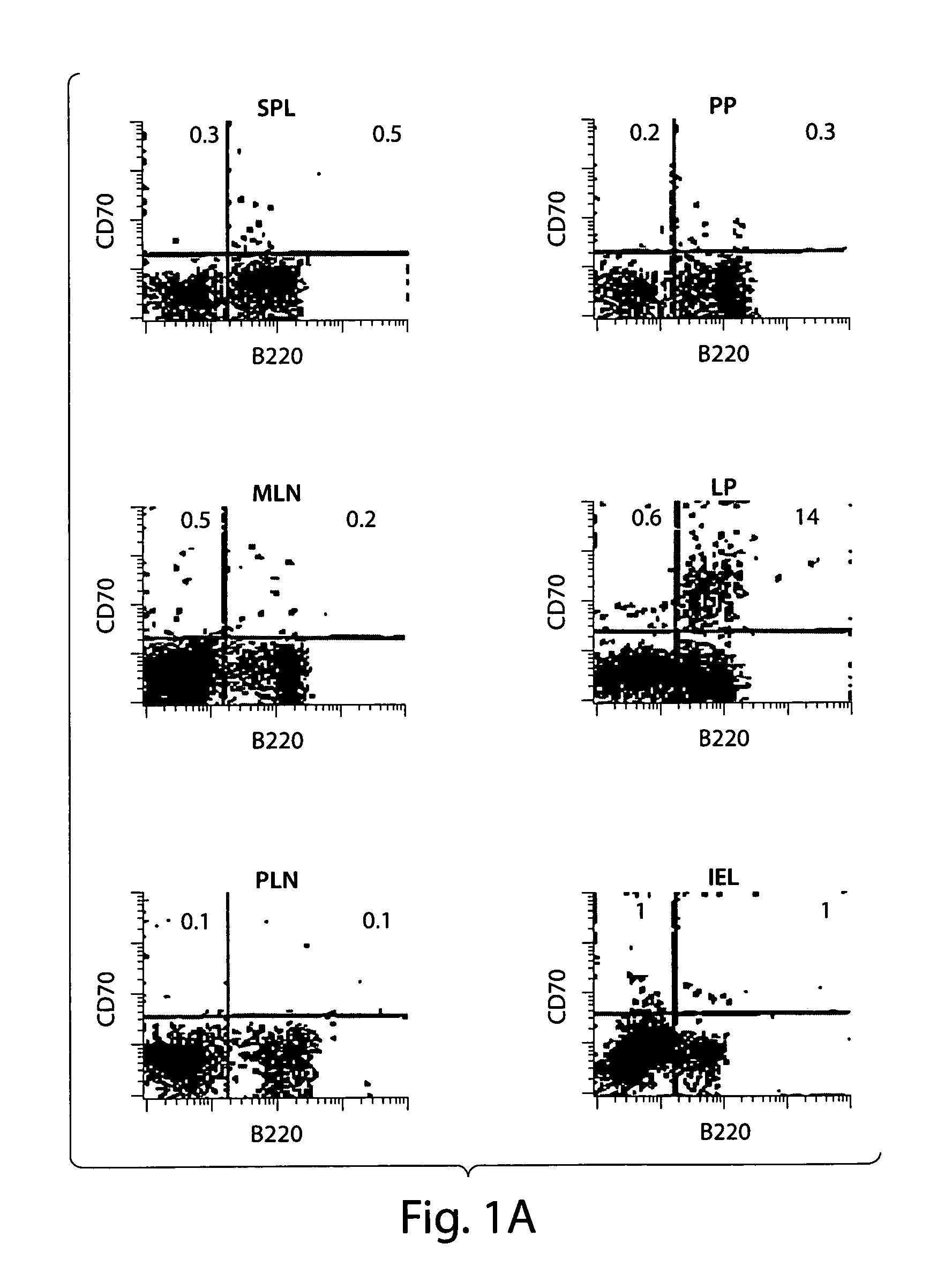
[0073]The constitutively activated phenotype and function of T cells in the intestinal mucosa is reminiscent of the persistent activation of T cells seen in CD70 Tg mice. CD70 expressing cells were tested to determine whether the cells are present in the intestinal mucosa in naïmice. A substantial portion of cells in the lamina propria compartment, but not in the other tissues tested, including Peyer's patch and mesenteric lymph nodes, were found to express CD70 as shown in FIG. 1A.
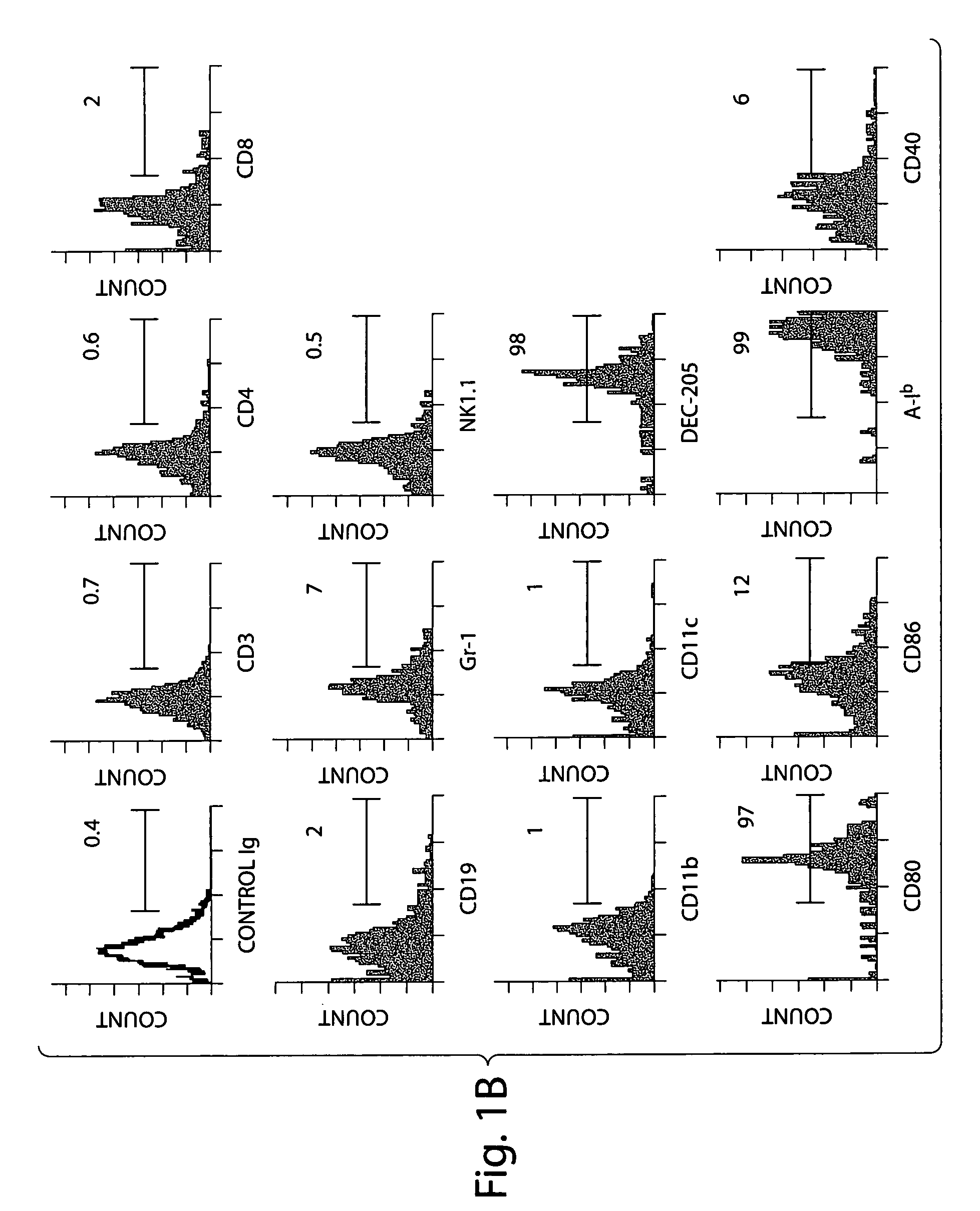
[0074]Surface CD70 antigen is expressed by activated B cells, mature dendritic cells, and to a lesser extent, T cells. The CD70 expressing cells were phenotyped in lamina propria in detail (see FIG. 1B). These cells were negative for CD3, CD4 or CD8 expression, indicating that they are not of T cell origin. The cells expressed B220, but were not B cells because they were CD19 negative. The cells also did not express the NK1.1 or G...
example 3
Antigen Presentation and Cytokine Secretion by APCLP Cells
[0075]APCLP cells were tested to determine whether these cells have antigen-presenting ability. Lamina propria cells from C57BL / 6 mice (H-2b) were first immunomagnetically depleted of B cells after staining with CD19 antibody, and the remaining cells were then stained with B220 antibody to positively select B220+ APCLP. The isolated cells were greater than 80% enriched for APCLP cells as assessed by staining with CD70 antibody. The ability of these cells to stimulate purified T cells from BALB / c mice (H-2d) was assessed. As shown in FIG. 2A, the APCLP cells were able to stimulate a potent alloresponse.
[0076]The cytokine secretion ability of APCLP cells was also evaluated. Cytokine production by immunomagnetically isolated APCLP cells was tested using a RayBio Mouse Cytokine Array I kit, which allows simultaneous detection of 22 cytokines at the protein level. As shown in FIG. 2B, ex vivo isolated APCLP cells from normal mice ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| body weight | aaaaa | aaaaa |
| adhesion | aaaaa | aaaaa |
| liquid absorption | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com