Endocardial lead removing apparatus
a technology of endocardial leads and endocardial leads, which is applied in the field of endocardial lead removal apparatus, can solve the problems of increasing electrode resistance, and unable to remove endocardial leads in the heart and venous path,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
first embodiment
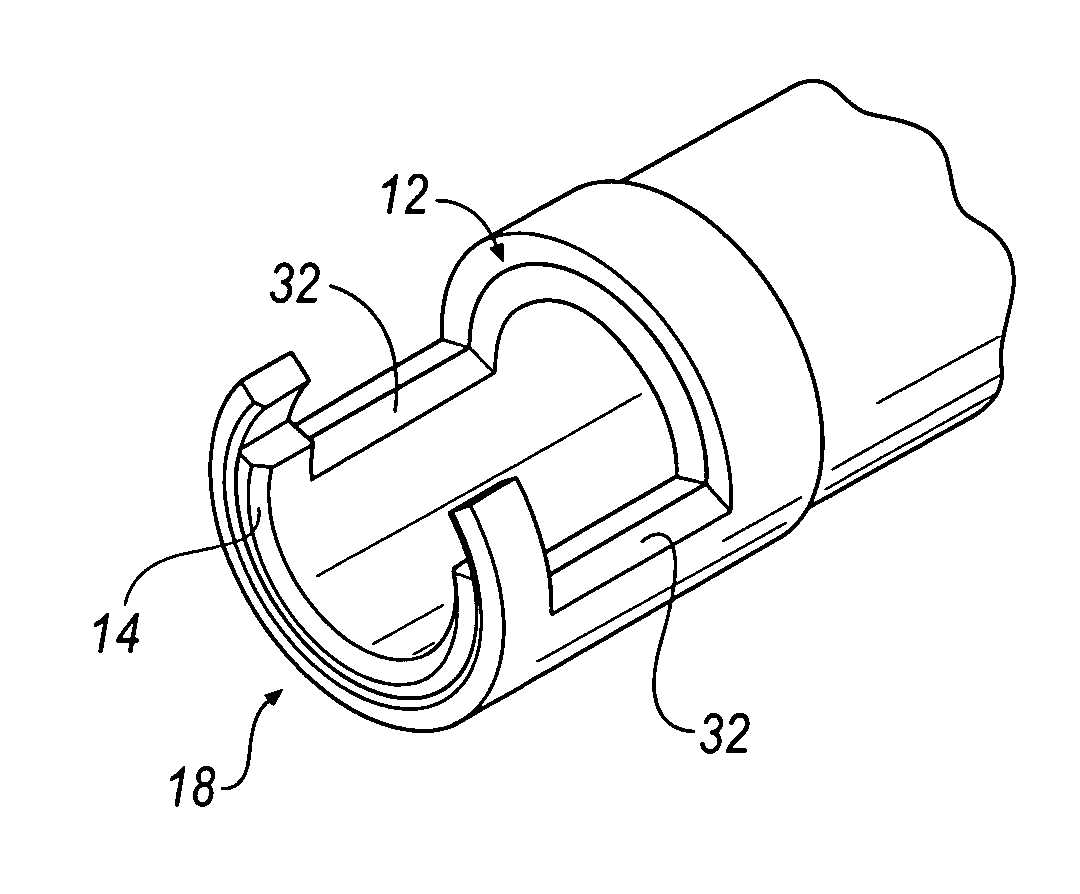
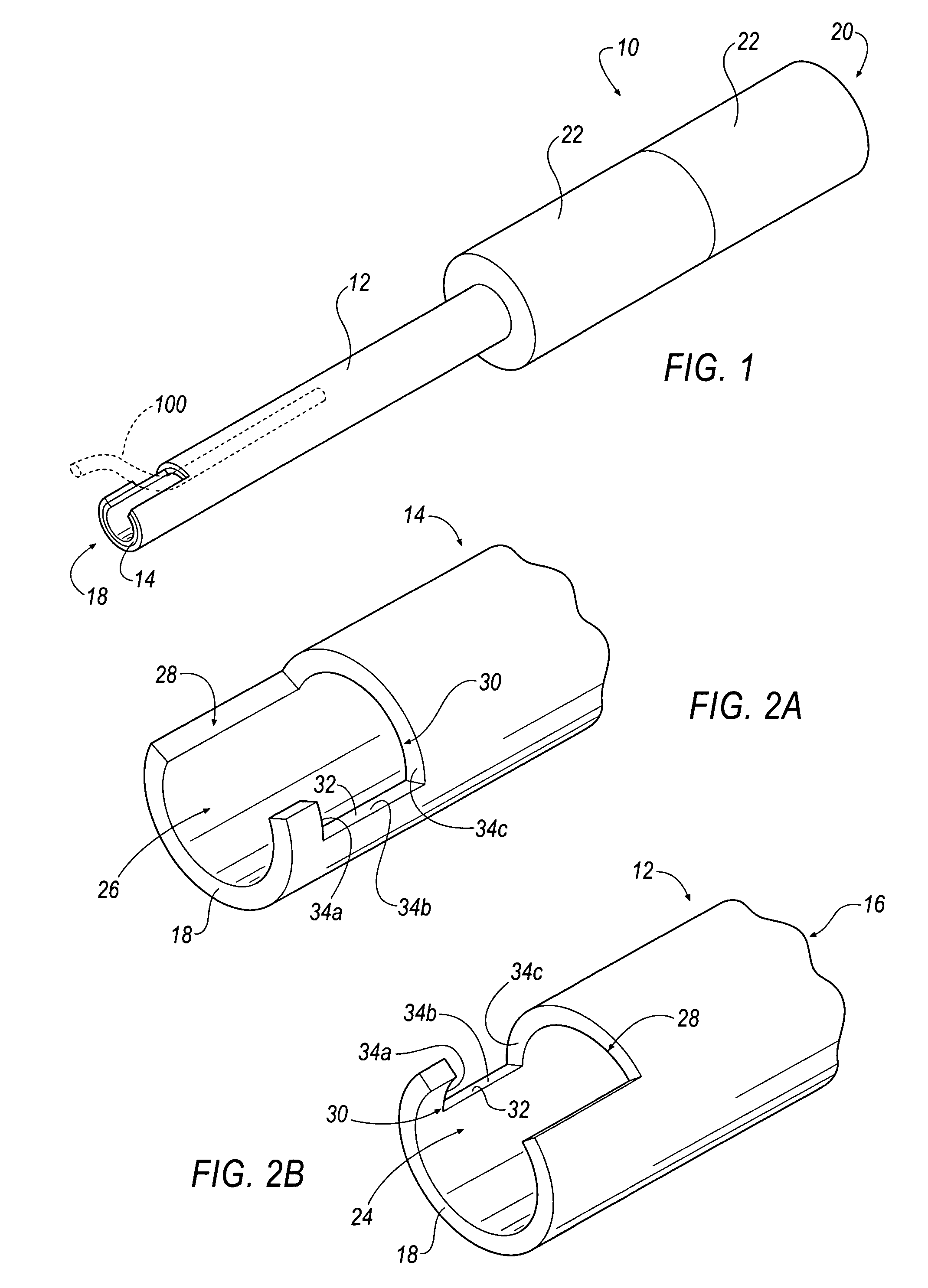
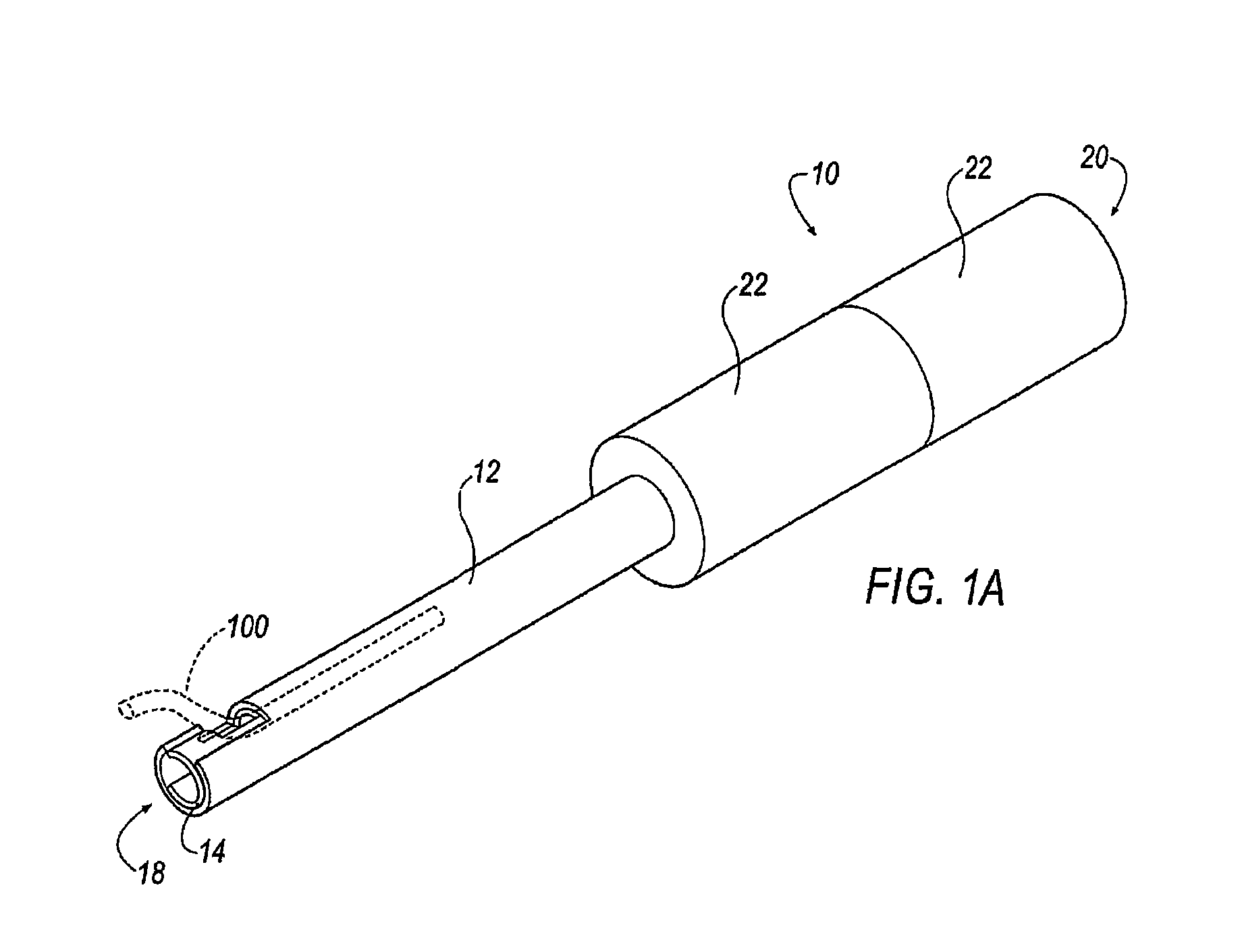
[0034]In operation, apparatus 10 of FIGS. 1-3 is inserted within a patient's chest cavity, blood vessel, or other anatomical part containing a lead (not shown) and receives a lead 100 (shown in phantom in FIG. 1) within the inner cavity 16 of the inner tubular member 14. Once the distal ends 18 of the apparatus 10 are positioned as near as possible to the embedded electrode (not shown) within the patient's body the lead 100 is received within the channels 24, 26. Each of the tubular members 12, 14 are independently rotatable and when rotated in opposite directions the lead 100 is captured in a completely enclosed window formed between the cutting surfaces 32 of each channel 24, 26 as shown in FIG. 1A. Additional torque applied to the handles 22 and further rotation of the members 12, 14 cuts through the lead 100. The severed portion of the lead 100 remains within the inner cavity 16 of the inner tubular member 14 and the apparatus 10 is removed from the patient's body.
[0035]Another ...
fifth embodiment
[0052]Referring to FIGS. 11-12, the apparatus 10 of the present invention is illustrated. The apparatus 10 includes a tubular member 512 defining a tubular wall 560 and a longitudinal axis A-A. The tubular member 512 is generally flexible and made from a plastic or polymer material; however, any material is contemplated by the present invention. The tubular member 512 and tubular wall 560 define an inner cavity 516. The inner cavity 516 is generally offset from the longitudinal axis A-A of the tubular member 512 in order to accommodate a blade 552 and adjustment mechanism 546 within the tubular wall 560.
[0053]The apparatus 10 includes the blade 552 disposed within the tubular wall 560 of the tubular member 512. The blade 552, by way of the adjustment mechanism 546, is moveable between a retracted position and an extended position. Further, the blade 552 includes a generally arcuate cutting surface 532.
[0054]The adjustment mechanism 546 of the fifth embodiment is pneumatically actuat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com