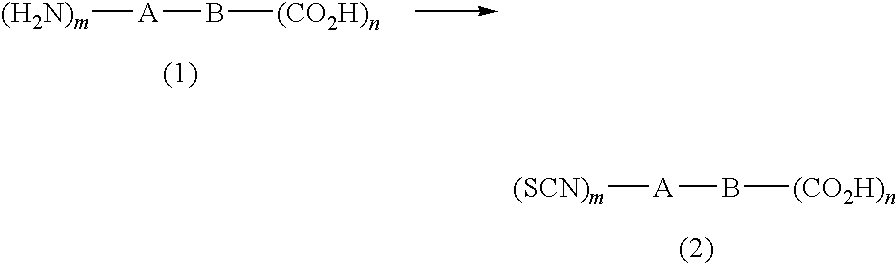
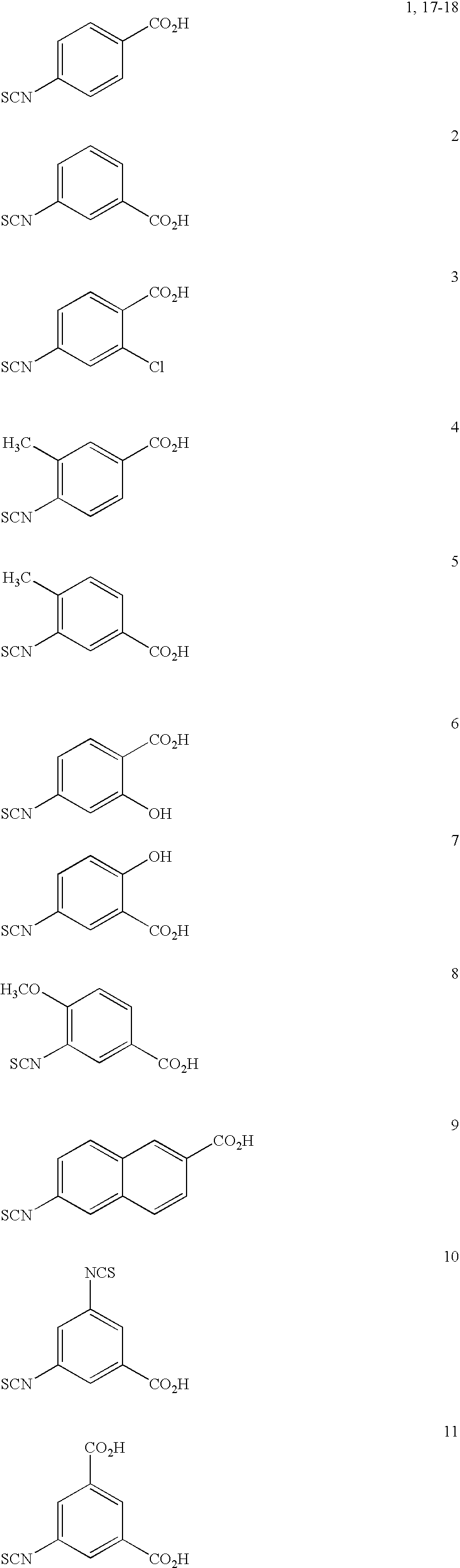
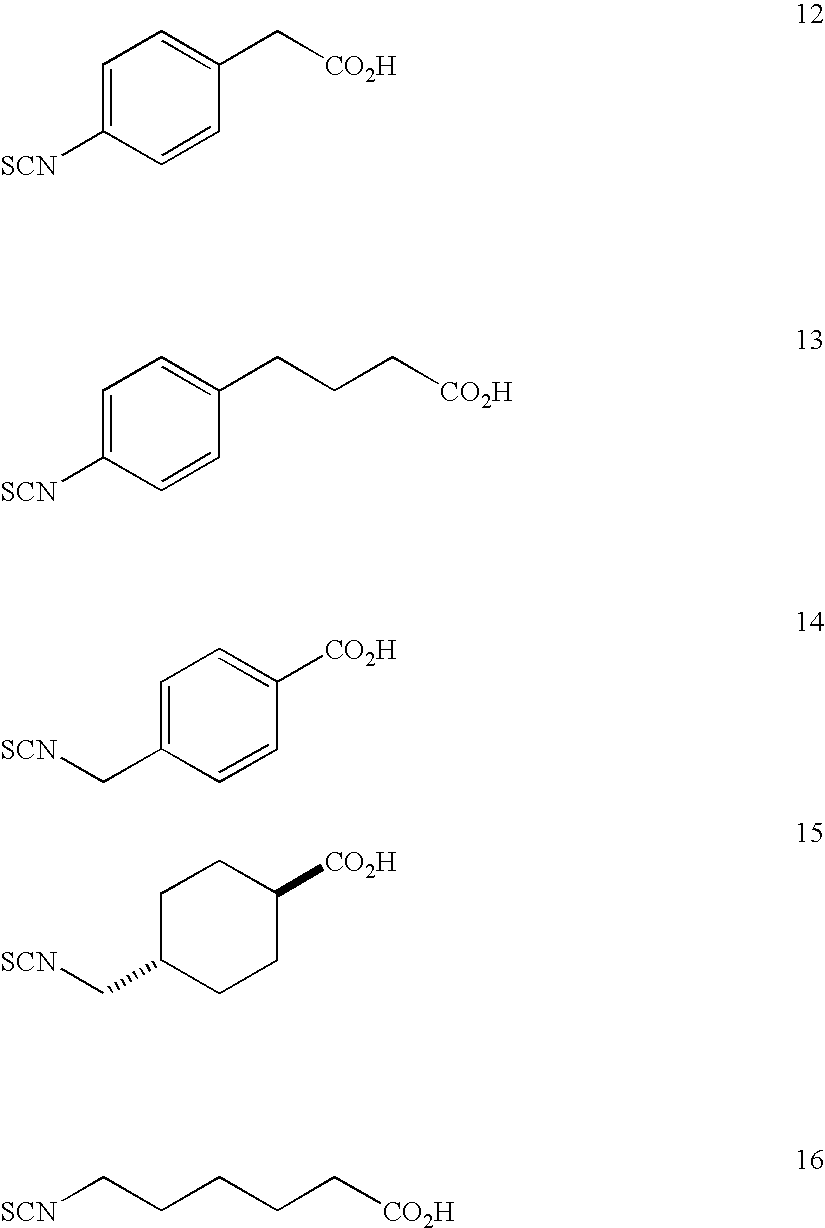
Method for producing isothiocyanate compound having carboxyl group
a technology of isothiocyanate and carboxyl group, which is applied in the field of producing isothiocyanate compound, can solve the problems of high cost of tetramethylthiuram disulfide to be used, inability to synthesize an isothiocyanate having an electron-drawing group, and high toxicity of thiophosgene itself, etc., and achieves high purity, high yield, and simple production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
4-Isothiocyanatobenzoic acid
[0114]Carbon disulfide (0.66 mL, 11 mmol) was added to a mixture comprising 4-aminobenzoic acid (0.50 g, 3.6 mmol), tetrahydrofuran (2.5 mL), water (2.5 mL) and triethylamine (1.3 mL, 9.1 mmol), followed by stirring at room temperature for 24 hours. To the obtained reaction mixture, a tetrahydrofuran (2.5 mL) solution of iodine (1.0 g, 4.0 mmol) was dropwise added over a period of 5 minutes at 0° C., followed by stirring at 0° C. for further two hours. Thereafter, 1M hydrochloric acid (3.6 mL) and sodium sulfite (91 mg, 0.72 mmol) were added and stirred. Then, ethyl acetate (15 mL) was added, and the organic layer was separated and concentrated under reduced pressure to dryness. To the residue, ethyl acetate (5 mL) and hexane (5 mL) were added and thoroughly mixed, and then, the insoluble matter was collected by filtration to obtain 4-isothiocyanatobenzoic acid as a colorless solid (0.65 g, yield: 100%, HPLC purity: 92%, HPLC retention time: 3.7 min). LC-...
example 2
3-Isothiocyanatobenzoic acid
[0117]Carbon disulfide (0.26 mL, 4.4 mmol) was added to a mixture comprising 3-aminobenzoic acid (0.20 g, 1.5 mmol), tetrahydrofuran (1.0 mL), water (1.0 mL) and triethylamine (0.51 mL, 3.6 mmol), followed by stirring at room temperature for 24 hours. To the obtained reaction mixture, a tetrahydrofuran (1.0 mL) solution of iodine (0.41 g, 1.6 mmol) was dropwise added over a period of 5 minutes at 0° C., followed by stirring at 0° C. for further two hours. Thereafter, 1M hydrochloric acid (1.5 mL) and sodium sulfite (38 mg, 0.30 mmol) were added and mixed. Then, ethyl acetate (6 mL) was added, and the organic layer was separated and concentrated under reduced pressure to dryness. To the residue, ethyl acetate (4 mL) and water (2 mL) were added, and the organic layer was concentrated under reduced pressure to dryness to obtain 3-isothiocyanatobenzoic acid as a cream-colored solid (0.25 g, yield: 96%, HPLC purity: 95%, HPLC retention time: 3.6 min). LC-MS ES...
example 3
4-Isothiocyanato-2-chlorobenzoic acid
[0118]Carbon disulfide (0.26 mL, 4.4 mmol) was added to a mixture comprising 4-amino-2-chlorobenzoic acid (0.26 g, 1.5 mmol), tetrahydrofuran (1.0 mL), water (1.0 mL) and triethylamine (0.51 mL, 3.6 mmol), followed by stirring at room temperature for 24.5 hours. To the obtained reaction mixture, a tetrahydrofuran (1.0 mL) solution of iodine (0.41 g, 1.6 mmol) was dropwise added over a period of 5 minutes at 0° C., followed by stirring at 0° C. for further two hours. Thereafter, 1M hydrochloric acid (1.5 mL) and sodium sulfite (38 mg, 0.30 mmol) were added and stirred. Then, ethyl acetate (6 mL) was added, and the organic layer was separated and concentrated under reduced pressure to dryness. To the residue, water (4 mL) and sodium hydrogencarbonate (0.14 g, 1.7 mmol) were added, and the insoluble matter was removed by filtration. To the filtrate, 1M hydrochloric acid (1.5 mL) and water (2.0 mL) were added, and the precipitated solid was collected...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com