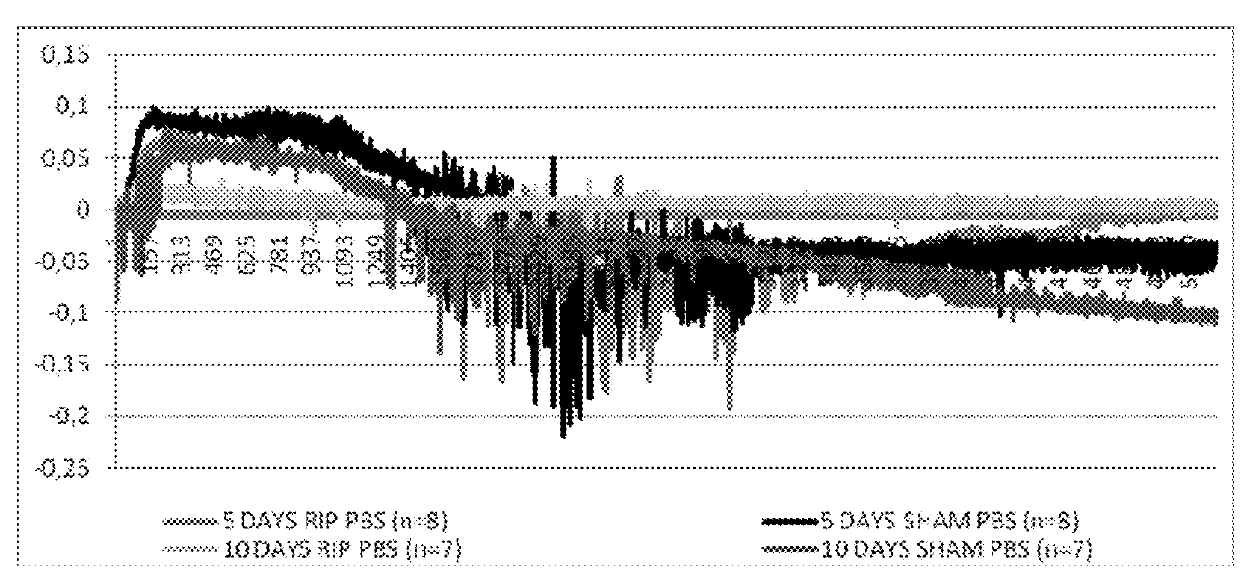
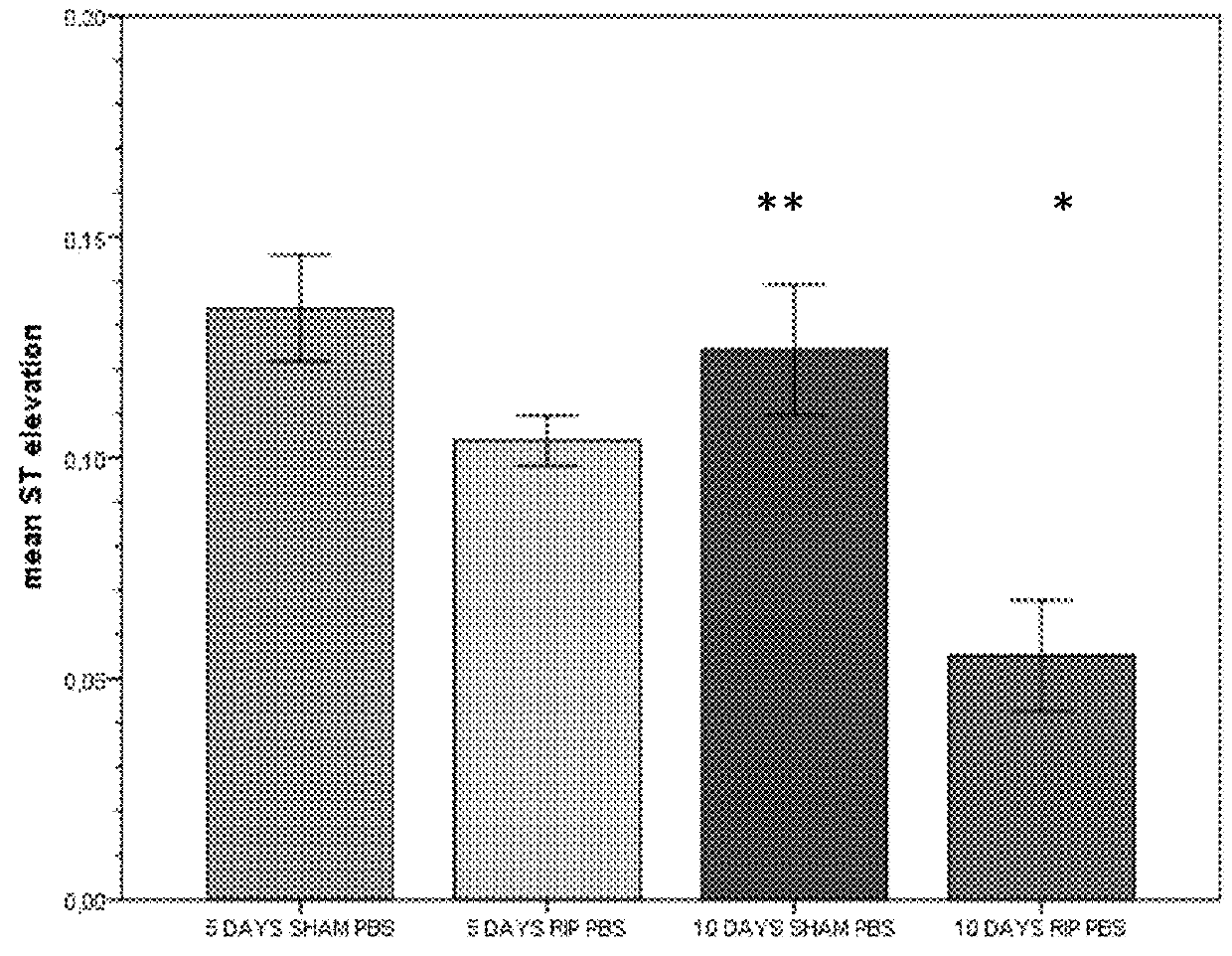
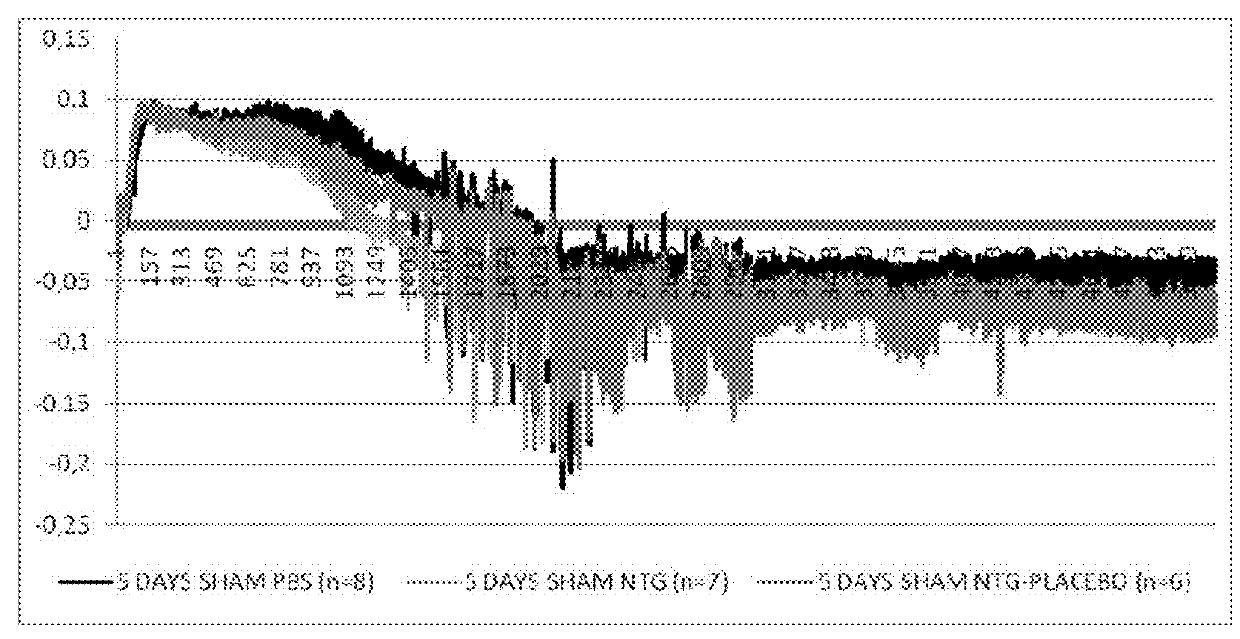
Use of stabilized granules containing glyceryl trinitrate for arteriogenesis
a technology of glyceryl trinitrate and stabilized granules, which is applied in the directions of pharmaceutical delivery mechanisms, organic active ingredients, and heterocyclic compound active ingredients, can solve the problems that nitroglycerin has not been used for curing the underlying disease or improving the prognosis, so as to reduce volatility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0228]
ContentsQuantity [g]GTN (5%) in diluent MCT1.033Triethyl citrate1.032Isomalt16.513Xylitol6.248Silicon dioxide (Aeroperl ®)0.207Total25.033GTN concentration0.207%
[0229]Triethyl citrate was mixed with GTN phlegmatized in a diluent of medium chain triglycerides (MCT). The solution was mixed well with the isomalt. Then xylitol and finally silicon dioxide were added and mixing was continued. 200-mg portions of the free-flowing powder were filled in stick packs and stored at 40° C. / 75% rel humidity for three months.
[0230]The GTN concentration was quantified after production and at various points during storage using HPLC analysis. The individual doses were dissolved in a suitable solvent to perform the analysis. The GTN was detected using a UV-VIS detector at a wavelength of 225 nm.
[0231]
GTN concentration following storage at 40° C. / 75% rel. humidityProductaccording to0 months2 weeks1 month3 monthsComp.0.391 mg0.079 mg0.065 mg*example 1Comp.0.407 mgn.c.0.305 mg0.245 mgexample 2Examp...
example 2
[0233]
ContentsQuantity [g]GTN (5%) in diluent MCT2.00TPGS1.00Magnesium aluminometasilicate2.50Isomalt44.5Total50.00GTN concentration0.20%
[0234]TPGS was melted at 50° C. and mixed with GTN concentrate in a diluent of MCT. While still warm, the mixture was blended well with the magnesium aluminometasilicate. Then isomalt was added and mixing was continued. 200-mg portions of the free-flowing powder were filled in stick packs and stored at 25° C. / 60% rel. humidity. The GTN concentration was quantified immediately after production and at various points during storage as disclosed under Example 1. The results are presented in the following table:
[0235]
Storage duration / temp.0 months3 months / 25° C.6 months / 25° C.GTN concentration0.400 mg0.398 mg0.392 mg
example 3
[0236]
ContentsQuantity [g]GTN (5%) in diluent MCT2.02Glycerol monocaprylocaprate Ph. Eur.0.52Magnesium aluminometasilicate1.50Isomalt45.99Total50.03GTN concentration0.20%
[0237]The glycerol monocaprylocaprate was melted at 40° C. and mixed with a GTN concentrate in MCT diluent. While still warm, the mixture was blended well with the magnesium aluminometasilicate. Then isomalt was added and mixing was continued. 200-mg portions of the free-flowing powder were filled in stick packs and stored at 40° C. / 75% rel humidity and at 25° C. / 60% rel. humidity. The GTN concentration was quantified immediately after production and at various points during storage as disclosed under Example 1. The results are presented in the following table:
[0238]
Storage duration / temp.0 months6 months / 25° C.6 months / 40° C.GTN concentration0.397 mg0.383 mg0.355 mg
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com