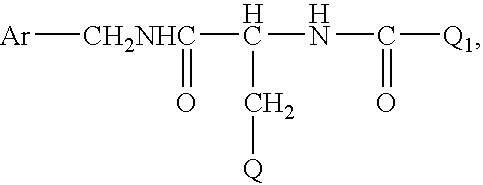
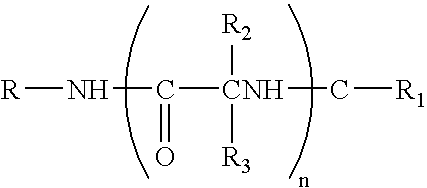
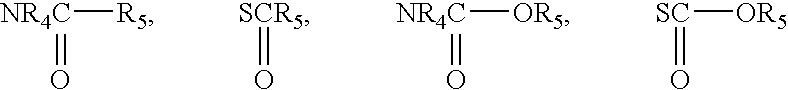Anticonvulsant enantiomeric amino acid derivatives
a technology of enantiomeric amino acids and anticonvulsant, which is applied in the direction of biocide, amide active ingredients, organic chemistry, etc., can solve the problems of disturbing side effects, no drug presently available is capable of achieving total seizure control, and a significant percentage of the population with epilepsy or related disorders
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
(R)-N-Benzyl-2-Acetamide-3-methoxypropionamide
Hydrochloric acid (8.00 g, 219.4 mmol) was passed into MeOH (250 mL) and then D-Serine (20.00 g, 190.3 mmol) was added. The reaction solution was heated at reflux (18 hours), benzylamine (81.6 mL, 761 mmol) was added and then the reaction was heated for an additional eighteen hours. The solvent was removed under reduced pressure, the insoluble salts filtered, and the excess benzylamine was removed under high vacuum (Kugelrohr). The residue was dissolved in water (100 mL), and the product was extracted with CHCl.sub.3 (8.times.200 mL). The organic layers were combined, dried (Na.sub.2 SO.sub.4), and the solvent was removed under reduced pressure. The residue was triturated with Et.sub.2 O (150 mL) and filtered to give 10.0 g (27%) of the product R-enriched N-benzyl 2-aminohydracrylamide, as a white solid: mp 74.degree.-78.degree. C.; [.alpha.].sub.D.sup.23 (c=1, MeOH)=1.6.degree., R.sub.f 0.30 (10% MeOH--CHCl.sub.3); .sup.1 H NMR (DMSO-d....
example 2
Another Synthesis of (R)-N-Benzyl 2-Acetamide-3-methoxy propionamide
(a) Improved Synthesis of (R)-N-Benzyl 2-Acetamidohydracrylamide
To a stirred AcOH (20 mL) suspension of D-serine (5.26 g, 50 mmol) was added Ac.sub.2 O (4.7 mL, 50 mmol), and then the reaction suspension was stirred at room temperature (24 hours). The ACOH was removed in vacuo to give an oily residue, and then THF (150 mL) was added to the residue. The THF suspension was cooled to -78.degree. C. under N.sub.2 and 4-methylmorpholine (11.0 mL, 100 mmol) was added. After stirring for two minutes, isobutyl chloroformate (13.0 mL, 100 mmol) was added leading to the precipitation of a white solid. The reaction was allowed to proceed for two additional minutes and then benzylamine (10.4 mL, 100 mmol) was added at -78.degree. C. The reaction mixture was allowed to stir at room temperature (30 minutes) and the 4-methylmorpholine hydrochloride salt was filtered. The organic layer was concentrated in vacuo. The product was pur...
example 3
R-N-(3-Fluorobenzyl)2-Acetamide-3-Methoxypropionamide
(a) R-N-(3-Fluorobenzyl)-2-Acetamide-hydracrylamide
Utilizing the procedure of Example 2(a) with the following amounts of D-serine (5.26 g, 50 mmol), Ac.sub.2 O (5.7 mL, 60 mmol), 4-methylmorpholine (11.0 mL, 100 mmol), isobutyl chloroformate (13.0 mL, 100 mmol) and substituting 3-fluorobenzylamine (11.8 mL, 100 mmol) for benzylamine, gave 4.20 g (33%) of the above compound as a white solid after purification: mp 137.degree.-138.degree. C.; [.alpha.].sub.D.sup.23 (c=1, MeOH)=+20.8.degree.; Rf0.32 (10% MeOH--CHCl.sub.3); IR (KBr) 3282, 3101, 2944, 1636, 1542, 1252, 1050, 779, 690 cm.sup.-1 ; .sup.1 H NMR (DMSO-d.sub.6) .delta.1.87 (s,C(O)CH.sub.3), 3.56-3.63 (m, CH.sub.2 OH), 4.29 (d, J=6.0 Hz, CH.sub.2 NH), 4.25-4.30 (m, CH), 4.95 (t, J=5.4 Hz, CH.sub.2 OH), 7.00-7.09 (m, 3 ArH), 7.29-7.30 (m, 1 ArH), 7.97 (d, J=8.1 Hz, NH), 8.44 (t, J=6.0 Hz, NH), addition of excess (R)-(-)-mandelic acid to a CDCl.sub.3 solution of this product ga...
PUM
| Property | Measurement | Unit |
|---|---|---|
| w/w | aaaaa | aaaaa |
| w/w | aaaaa | aaaaa |
| w/w | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com